
A. KESH HEBBAR, M.D., AND WILLIAM J. HUESTON, M.D.
A more recent article on common types of supraventricular tachycardia is available.
Am Fam Physician. 2002;65(12):2479-2487
This is part I of a two-part article on common arrhythmias. Part II, “Ventricular Arrhythmias and Arrhythmias in Special Populations,” appears on page 2491 of this issue.
Family physicians frequently encounter patients with symptoms that could be related to cardiac arrhythmias, most commonly atrial fibrillation or supraventricular tachycardias. The initial management of atrial fibrillation includes ventricular rate control to provide adequate cardiac output. In patients with severely depressed cardiac output and recent-onset atrial fibrillation, immediate electrical cardioversion is the treatment of choice. Hemodynamically stable patients with atrial fibrillation for more than two days or for an unknown period should be assessed for the presence of atrial thrombi. If thrombi are detected on transesophageal echocardiography, anticoagulation with warfarin for a minimum of 21 days is recommended before electrical cardioversion is attempted. Patients with other supraventricular arrhythmias may be treated with adenosine, a calcium channel blocker, or a short-acting beta blocker to disrupt reentrant pathways. When initial medications are ineffective, radiofrequency ablation of ectopic sites is an increasingly popular treatment option.
Heart palpitations and cardiac arrhythmias are common problems encountered by family physicians. Patients may present with acute cardiac rhythm abnormalities. Although these arrhythmias are usually benign, they can indicate significant underlying heart disease. More often, patients have chronic arrhythmias, such as atrial fibrillation, that may require treatment to reduce the risk of future complications. The challenges for the family physician are to determine which arrhythmias are benign and which indicate probable cardiac malfunction, and to manage recurrent or chronic rhythm abnormalities.
This two-part article reviews common atrial and ventricular arrhythmias, with a focus on initial management decisions. Part I discusses supraventricular arrhythmias. Part II discusses ventricular arrhythmias and the management of rhythm abnormalities in special populations, including pregnant women, athletes, and children.

Atrial Fibrillation
Atrial fibrillation is the most common cardiac arrhythmia family physicians are likely to encounter. This rhythm abnormality affects 3 to 5 percent of patients more than 60 years of age 1 and becomes increasingly common with advancing age. The median age of patients with atrial fibrillation is 75 years, and the prevalence of the arrhythmia doubles every 10 years after the age of 55. 2 , 3 In the United States, atrial fibrillation is estimated to affect almost 9 percent of patients more than 75 years of age. 2
Most risk factors for atrial fibrillation are associated with structural or ischemic heart disease. Risk factors include hypertension, left ventricular hypertrophy, dilated and restrictive cardiomyopathies, coronary artery disease, chronic obstructive pulmonary disease, and diabetes in women. 1
The annual risk of stroke in patients with atrial fibrillation and normal valve function has been reported to be 4.5 percent per year. 4 Anticoagulation with warfarin (Coumadin) reduces the risk by about two thirds. 4 The mortality rate for stroke in patients with atrial fibrillation is approximately twice as high as the rate in patients without this rhythm abnormality. 5 Although anticoagulation is contraindicated in some elderly patients, a study in Great Britain 6 found that about 60 percent of patients identified in community screenings as having atrial fibrillation were eligible for, and would benefit from, this treatment.
The first step in managing a patient with atrial fibrillation is to decide whether there is a high likelihood of safe conversion to sinus rhythm or whether the patient should be allowed to remain in atrial fibrillation. A patient with recent onset of atrial fibrillation (within the previous 12 months) and no evidence of enlargement of the left atrium has a greater chance of achieving and maintaining sinus rhythm. If the arrhythmia is long-standing and the patient is not a suitable candidate for rate cardioversion, initial treatment should focus on ventricular rate control, with consideration given to long-term stroke prophylaxis.
Restoration of Sinus Rhythm
Patients who present within 48 hours of the onset of new atrial fibrillation are candidates for cardioversion with a low risk of embolism. Conversion to sinus rhythm can be attempted by electrical shock or with antiarrhythmic drugs. Patients who have been in atrial fibrillation for more than 48 hours or for an undetermined period are more likely to have atrial thrombi and may develop emboli with immediate electrical or medical (pharmacologic) cardioversion.
Atrial thrombi are not evident on transthoracic echocardiograms, but they can been seen on transesophageal echocardiograms. 7 If the transesophageal echocardiogram reveals thrombi, anticoagulation is recommended before cardioversion is attempted. Anticoagulation can be accomplished using warfarin, with the dosage adjusted to achieve an International Normalized Ratio (INR) between 2.0 and 3.0 for a minimum of 21 days. 8
If the transesophageal echocardiogram does not show thrombi on multiplane views, cardioversion can be attempted. Short-term anticoagulation with heparin should be started before the procedure, and warfarin therapy should be initiated after cardioversion. 8
When rhythm conversion is indicated, it can be accomplished using direct-current cardioversion or pharmacologic therapy. Synchronized cardioversion is currently considered the treatment of choice for the restoration of sinus rhythm and, in appropriately selected patients, has a success rate of at least 80 percent. 4 Cardioversion is also indicated in patients with hypotension, angina, heart failure, or other evidence of severe compromise caused by atrial fibrillation. 5
Medical cardioversion of atrial fibrillation may be achieved with class IA drugs (quinidine, disopyramide [Norpace], procainamide [Procanbid]) or with amiodarone (Cordarone). In the past, quinidine was frequently used for both cardioversion and maintenance of sinus rhythm in patients who had undergone electrical cardioversion. However, because of the proarrhythmic action of class IA agents and their detrimental effects on left ventricular function, these drugs are now used less often than amiodarone for primary therapy of atrial fibrillation. 4
Amiodarone therapy is successful in 86 percent of patients who have had atrial fibrillation for less than two years. 4 , 9 Treatment is also effective in 40 to 60 percent of patients with long-standing atrial fibrillation that has been resistant to other agents and to electrical cardioversion. 4 Amiodarone can be given in a dosage of 200 mg a day, which is lower than the dosages that have been associated with thyroid abnormalities and pulmonary fibrosis. Although there is little risk of toxicity when amiodarone is given in a low dosage, it is prudent to monitor patients for the development of thyroid, pulmonary, hepatic, and cardiac side effects.
Findings on the usefulness of various agents for the conversion of atrial fibrillation, based on the evidence-based practice program of the Agency for Healthcare Research and Quality, are summarized in Table 1 . 10 Although drugs such as digitalis preparations and sotalol (Betapace) are sometimes used for rate control, they are not effective for converting atrial fibrillation to sinus rhythm. 10 , 11
If external electrical cardioversion is unsuccessful and antiarrhythmic drug therapy fails, other measures can be used. However, these approaches are usually reserved for use in patients who cannot tolerate atrial fibrillation and patients who have associated systolic dysfunction. Techniques include internal electrical cardioversion through the application of electrical current to pulmonary veins via a transcatheter cathode 4 and radiofrequency ablation of the atrioventricular node with insertion of a ventricular pacemaker. 12 In addition, an implantable atrial defibrillator can be used to provide rapid cardioversion in patients with atrial fibrillation that cannot be controlled with medications. 13
Rate Control in Chronic Atrial Fibrillation
In patients in whom rhythm conversion is not indicated or those who have new-onset atrial fibrillation with a rapid ventricular response, treatment may be needed to control the ventricular rhythm. Excessive ventricular rates may result in diminished cardiac output because of poor filling time, and in ischemia because of increased myocardial oxygen demand. Medications used for ventricular rate control in patients with atrial fibrillation are listed in Table 2 . 14
Acute management of ventricular rates can usually be achieved with intravenously administered diltiazem (Cardizem), given in an initial bolus of 15 to 20 mg (0.25 mg per kg) over two minutes, or with an intravenously administered beta blocker such as propranolol (Inderal), given in a dose of 0.5 to 1 mg (up to 3 to 5 mg if needed).
A number of medications, including calcium channel blockers, beta blockers, and digoxin (Lanoxin), are effective for maintaining ventricular rates within acceptable ranges. Because calcium channel blockers are associated with better exercise tolerance, they may be preferable to beta blockers. 15 Digoxin is associated with a high degree of exercise intolerance; therefore, it should be reserved for use in patients who are relatively immobile, who cannot tolerate other treatment options, or who have significant ventricular dysfunction.
Paroxysmal Supraventricular Tachycardias
Based on duration, supraventricular tachycardias are usually categorized as paroxysmal, persistent, or chronic. Paroxysmal supraventricular tachycardia (PSVT) is the most common of these arrhythmias and the one that is most often encountered in the primary care setting. Longer-duration supraventricular tachycardias can be treated similarly to PSVT, but cardiology consultation is often required to identify the electrophysiologic mechanism responsible for sustaining the arrhythmia. In contrast to ventricular tachycardias (discussed in part II of this article) and atrial fibrillation, PSVT is usually a narrow-complex tachycardia with a regular rate.
Atrioventricular Nodal Reentry Causing PSVT
Atrioventricular nodal reentry, the most common mechanism of PSVT, occurs when two pathways exist with different conduction rates. A premature atrial complex that is blocked in the fast pathway and redirected through the slow pathway usually triggers the tachycardia ( Figure 1 ) . The electrical signal proceeds down the slow pathway and then reenters the fast pathway in a retrograde direction. By the time the signal has propagated down the slow pathway and back around on the fast pathway, the slow pathway is no longer refractory and is ready to conduct the signal again, completing a continuous circuit.
Reentrant tachycardias usually produce a narrow-complex tachycardia with no discernible P wave. The rate is usually between 160 and 190 beats per minute. In a less common form of atrioventricular nodal reentrant tachycardia, the circulating wavefront proceeds in an antegrade fashion down the fast pathway and in a retrograde fashion up the slow pathway. In this form, inverted P waves ( Figure 2 ) are clearly visible in lead II of the electrocardiogram (ECG).
It is important to note that atrioventricular nodal reentrant tachycardia can result in a wide-complex tachycardia if the patient has preexisting bundle branch block.
Accessory Pathways Causing PSVT
Accessory pathways (Wolff-Parkinson-White syndrome) and other bypass tracts can cause PSVT. In patients with Wolff-Parkinson-White syndrome, a shortened PR interval and a slurred upstrike to the QRS complex “delta wave” on the resting ECG indicate the presence of an accessory pathway ( Figure 3 ) .
It should be noted that the resting ECG may be normal in some patients with Wolff-Parkinson-White syndrome, because of the inability of the accessory pathway to conduct in the antegrade direction. The usual mechanism of PSVT in this setting is antegrade conduction down the normal pathways through the atrioventricular node and retrograde conduction through the accessory pathway.
The ECG in an atrial arrhythmia with an accessory pathway usually shows a narrow-complex tachycardia at rates of 160 to 240 beats per minute. Delta waves are absent because the normal pathways are used for ventricular activation. Inverted P waves may be seen in the inferior leads. In a much less common form of PSVT, antegrade conduction is down the bypass tract and results in a wide-complex tachycardia.
Increased Automaticity Causing PSVT
Increased automaticity usually occurs when the atrium is enlarged, as in patients with chronic lung disease, congestive heart failure, or electrolyte and acid-base disturbances. Usually, the stretched atria fire irregularly, producing multiple premature beats that emanate from different areas of the atria. Because the foci for the ectopic beats are in multiple sites, the P waves vary in morphology, giving rise to the term “multifocal atrial tachycardia.”
The diagnosis of multifocal atrial tachycardia depends on the identification of an irregular rhythm with three or more different P-wave morphologies. The rate is usually between 130 and 180 beats per minute. Treatment is directed at correcting the underlying cause. Antiarrhythmic drugs are usually not helpful.
In most patients, PSVT is benign and self-limited. However, some patients can have angina, hypotension, and intense anxiety. The first step in the management of PSVT is to determine whether the patient is hemodynamically stable. If PSVT is sustained and there is any indication of instability (i.e., angina, shortness of breath, decreased level of consciousness, hypotension, or congestive heart failure), electrical cardioversion should be performed urgently.
If the symptoms are restricted to discomfort (e.g., palpitations and anxiety), conservative measures should be applied. Conservative management of PSVT can include both nonpharmacologic and pharmacologic measures ( Table 3 ) . 16
Vagal maneuvers to increase parasympathetic tone and slow conduction through the atrioventricular node should be the first approach. Patients should be taught some of these maneuvers for use in future episodes. They should also be instructed to avoid inciting factors, such as caffeine, tobacco, alcohol, pseudoephedrine, and stress. Carotid sinus massage can be attempted, but its role hasbecome more limited because of the effectiveness of drug therapy and the risk of embolism from carotid pressure in some patients.
The goal of pharmacologic management is to slow or block atrioventricular nodal conduction. Agents used for this purpose include adenosine (Adenocard), calcium channel blockers (verapamil [Calan] or diltiazem), and beta blockers (e.g., esmolol [Brevibloc]).
Adenosine is an ultra–short-acting agent that is cleared quickly (half-life of 1 to 6 seconds). This agent is given intravenously in an initial dose of 6 mg, which is followed by one or two 12-mg boluses. Adenosine works by reducing conductance along the slow antegrade pathway. Side effects include flushing, dyspnea, and chest pain. Because of the short half-life of adenosine, these effects are usually very brief and do not ordinarily result in complications.
One advantage of adenosine is that it lacks the negative inotropic effects of calcium channel blockers. Adenosine can also decrease the sinus rate transiently and produce a “rebound” sinus tachycardia. Adenosine should not be used in patients with heart transplants, because such patients may be too sensitive to its effects. 17
Calcium channel blockers can also be used to disrupt a reentrant pathway. Verapamil can be given in a 5- to 10-mg bolus over 2 minutes, followed by 10 mg in 15 to 30 minutes if the initial dose does not convert the arrhythmia. 18 Verapamil and other calcium channel blockers should not be used in patients with an undiagnosed wide-complex tachycardia, because of the risk of fatal hypotension or ventricular fibrillation if the arrhythmia is actually ventricular tachycardia and not PSVT. 19
Intravenously administered diltiazem is also effective. 20 Initial treatment consists of a bolus of 0.25 mg per kg administered over two minutes. A repeat bolus of 0.35 mg per kg given over two minutes can be administered 15 minutes later.
Esmolol, a short-acting beta blocker, can be given in an intravenous bolus of 0.5 mg per kg over 1 minute or in an infusion at a rate of 0.5 mg per kg per minute after an initial loading dose of 0.5 mg per kg. An advantage of esmolol over other beta blockers is its short half-life (four to five minutes), compared with the much longer half-lives (three hours or more) of most other beta blockers. Because of a similar depressive effect on left ventricular contractility, esmolol should be used with caution if initial treatment with a calcium channel blocker is not successful.
Other antiarrhythmic drugs, including quinidine, procainamide, flecainide (Tambocor), and amiodarone, may be used in patients who do not respond to initial medications. However, selective radiofrequency ablation is rapidly becoming the treatment of choice in this situation.
Long-term control of recurrent PSVT caused by atrioventricular nodal reentry may be achieved with pharmacologic therapy or radiofrequency ablation. Patients who have infrequent, well-tolerated recurrences may manage these episodes with self-administered physiologic maneuvers.
Radiofrequency ablation is now used early in the management of patients with PSVT caused by an accessory pathway (Wolff-Parkinson-White syndrome), atrioventricular nodal reentrant tachycardia, or atrial tachycardia. 21 The success rate for radiofrequency ablation is 95 percent in patients with an accessory pathway or atrioventricular nodal reentrant tachycardia, and approximately 80 percent in patients with atrial tachycardia. 21
Other Atrial Arrhythmias
Sinus arrhythmia.
Sinus arrhythmia is usually a normal event in young persons and athletes. In fact, it occurs with such high frequency that it may considered a normal variant rather than a true arrhythmia.
There are two forms of sinus arrhythmia. In the “respiratory” form, the RR interval shortens during inspiration and slows during expiration. Breath-holding eliminates the variation. In the “nonrespiratory” form, the same phasic variation is seen in the RR interval but is not related to respirations. This form of sinus arrhythmia occurs in elderly patients, patients with digoxin overdose, and patients with increased intracranial pressure.
Sinus arrhythmia is usually asymptomatic. Sometimes, however, the long pauses can cause dizziness or syncope. Treatment is usually unnecessary.
WANDERING ATRIAL PACEMAKER
Patients with wandering atrial pacemaker are usually not symptomatic. The condition is most often an isolated finding on the ECG and requires no treatment. Sometimes it is noted on physical examination as an irregularly irregular rhythm.
With wandering atrial pacemaker, the ECG shows variable P-wave morphology and PR intervals. The atrial impulses conduct in a 1:1 fashion and usually control the rhythm for several beats before shifting to another focus. The normal heart rate in wandering atrial pacemaker differentiates this condition from multifocal atrial tachycardia.
PREMATURE ATRIAL COMPLEXES
A premature atrial complex is generated from an ectopic focus in the atria. Therefore, the P wave is usually different in morphology from the usual sinus P wave. The impulse conducts along the normal pathways, generating a narrow QRS complex followed by a pause. Sometimes the premature atrial complex is not conducted and can mimic heart block ( Figure 4 ) .
Premature atrial complexes are found in a variety of settings, including the excessive consumption of caffeine or alcohol and the use of sympathomimetic drugs. These complexes can also be present in patients with structural heart disease.
Patients with premature atrial complexes are usually asymptomatic and require no treatment. A beta blocker given in a low dosage can be tried in patients with uncomfortable symptoms, but no studies of efficacy have been reported. Patients should be counseled to decrease their intake of caffeine, tobacco, and alcohol, and their use of over-the-counter sympathomimetic substances, which are often present in cold medicines and weight-loss preparations.
It is important to note that premature atrial complexes sometimes precipitate supraventricular tachycardia, atrial flutter, or atrial fibrillation.
Sinus Nodal Arrhythmias
Sinus pause and sinoatrial exit block.
Sinus pause or arrest occurs when the sinoatrial node fails to discharge. The ECG shows a pause in the sinus rhythm, with no preceding P wave. Patients usually have no symptoms, but if the pause is prolonged, they may have lightheadedness, palpitations, syncope, and falls. In sinus arrest, the length of the pause has no relationship to the PP interval. Sinoatrial exit block is recognized by the pauses being multiples of PP intervals.
Sinus node dysfunction is usually caused by drugs such as digoxin, quinidine, or procainamide. It can also be caused by ischemia, myocarditis, or fibrosis.
From a therapeutic standpoint, it is probably not important to distinguish between sinus arrest and sino-atrial exit block. Both can occur in well-trained athletes 22 and can be a factor in sick sinus syndrome. 23
SICK SINUS SYNDROME
The term “sick sinus syndrome” encompasses a number of abnormalities, including sinus bradycardia, sinus arrest or exit block, combinations of sinoatrial and atrioventricular nodal conduction disturbances, and atrial tachyarrhythmias. More than one of these arrhythmias may be recorded in the same patient (bradycardia-tachycardia syndrome).
The abnormalities in sick sinus syndrome are usually due to ischemia, fibrosis, or drug-induced or autonomic dysfunction. Signs and symptoms are related to cerebral hypoperfusion and reduced cardiac output.
Treatment of recurrent symptomatic bradycardia or prolonged pauses requires implantation of a permanent pacemaker. 24
Levy S. Epidemiology and classification of atrial fibrillation. J Cardiovasc Electrophysiol. 1998;9(8 suppl):S78-82.
Ryder KM, Benjamin EJ. Epidemiology and significance of atrial fibrillation. Am J Cardiol. 1999;84(9A):R131-8.
Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840-4.
Golzari H, Cebul RD, Bahler RC. Atrial fibrillation: restoration and maintenance of sinus rhythm and indications for anticoagulation therapy. Ann Intern Med. 1996;125:311-23.
Pritchett EL. Management of atrial fibrillation. N Engl J Med. 1992;326:1264-71.
Sudlow M, Thomson R, Thwaites B, Rodgers H, Kenny RA. Prevalence of atrial fibrillation and eligibility for anticoagulants in the community. Lancet. 1998;352:1167-71.
Falk RH. Atrial fibrillation. N Engl J Med. 2001;344:1067-78.
Manning WJ, Silverman DI, Keighley CS, Oettgen P, Douglas PS. Transesophageal echocardiographically facilitated early cardioversion from atrial fibrillation using short-term anticoagulation: final results of a prospective 4.5-year study. J Am Coll Cardiol. 1995;25:1354-61.
Santos AL, Aleixo AM, Landieri J, Luis AS. Conversion of atrial fibrillation to sinus rhythm with amiodarone. Acta Med Port. 1979;1:15-23.
Management of new onset atrial fibrillation. Summary, evidence report/technology assessment: no. 12. Rockville, Md.: Agency for Healthcare Research and Quality, May 2000; AHRQ publication no. 00-E006. Retrieved April 23, 2002, from www.ahcpr.gov/clinic/epcsums/atrialsum.htm .
Falk RH, Knowlton AA, Bernard SA, Gotlieb NE, Battinelli NJ. Digoxin for converting recent-onset atrial fibrillation to sinus rhythm. A randomized, double-blinded trial. Ann Intern Med. 1987;106:503-6.
Pappone C, Rosanio S, Oreto G, Tocchi M, Gugliotta F, Vicedo-mini G, et al. Circumferential radiofrequency ablation of pulmonary vein ostia: a new anatomic approach for curing atrial fibrillation. Circulation. 2000;102:2619-28.
Swerdlow CD, Schsls W, Dijkman B, Jung W, Sheth NV, Olson WH, et al. Detection of atrial fibrillation and flutter by a dual-chamber implantable cardioverter-defibrillator. For the Worldwide Jewel AF Investigators. Circulation. 2000;101:878-85.
Physicians' desk reference. 56th ed. Montvale, N.J.: Medical Economics, 2002.
Segal JB, McNamara RL, Miller MR, Kim N, Goodman SN, Powe NR, et al. The evidence regarding the drugs used for ventricular rate control. J Fam Pract. 2000;49:47-59.
Myerburg RJ, Kessler KM, Castellanos A. Recognition, clinical assessment, and management of arrhythmias and conduction disturbances. In: Alexander RW, Schlant RC, Fuster V, eds. Hurst's The heart, arteries and veins. 9th ed. New York: McGraw-Hill, Health Professions Division, 1998:873–942.
O'Nunain S, Jennison S, Bashir Y, Garratt C, McKenna W, Camm AJ. Effects of adenosine on atrial repolarization in the transplanted human heart. Am J Cardiol. 1993;71:248-51.
Rinkenberger RL, Prystowsky EN, Heger JJ, Troup PJ, Jackman WM, Zipes DP. Effects of intravenous and chronic oral verapamil administration in patients with supraventricular tachyarrhyth-mias. Circulation. 1980;62:996-1010.
Stewart RB, Bardy GH, Greene HL. Wide complex tachycardia: misdiagnosis and outcome after emergent therapy. Ann Intern Med. 1986;104:766-71.
Betriu A, Chaitman BR, Bourassa MG, Brevers G, Scholl JM, Bruneau P, et al. Beneficial effect of intravenous diltiazem in the acute management of paroxysmal supraventricular tach-yarrhythmias. Circulation. 1983;67:88-94.
Morady F. Radio-frequency ablation as treatment for cardiac arrhythmias. N Engl J Med. 1999;340:534-44.
Bjornstad H, Storstein L, Meen HD, Hals O. Ambulatory electrocardiographic findings in top athletes, athletic students and control subjects. Cardiology. 1994;84:42-50.
Wu DL, Yeh SJ, Lin FC, Wang CC, Cherng WJ. Sinus automaticity and sinoatrial conduction in severe symptomatic sick sinus syndrome. J Am Coll Cardiol. 1992;19:355-64.
Haywood GA, Katritsis D, Ward J, Leigh-Jones M, Ward DE, Camm AJ. Atrial adaptive rate pacing in sick sinus syndrome: effects on exercise capacity and arrhythmias. Br Heart J. 1993;69:174-8.
Continue Reading
More in afp, more in pubmed.
Copyright © 2002 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.
Wandering Atrial Pacemaker EKG Interpretation with Rhythm Strip
Ekg features, wandering atrial pacemaker ekg interpretation example.
This website is only for professional medical education. Contact your doctor for medical care. 2024 © MedEdu LLC. All Rights Reserved. Terms & Conditions | About Us | Privacy | Email Us
We have a new app!
Take the Access library with you wherever you go—easy access to books, videos, images, podcasts, personalized features, and more.
Download the Access App here: iOS and Android . Learn more here!
- Remote Access
- Save figures into PowerPoint
- Download tables as PDFs

Wandering Atrial Pacemaker
- Download Chapter PDF
Disclaimer: These citations have been automatically generated based on the information we have and it may not be 100% accurate. Please consult the latest official manual style if you have any questions regarding the format accuracy.
Download citation file:
- Search Book
Jump to a Section
Key features, clinical presentation, diagnostic evaluation, ongoing management.
- Full Chapter
- Supplementary Content
ESSENTIALS OF DIAGNOSIS
Progressive cyclic variation in P-wave morphology
Heart rate 60–100 bpm
Variation of P-wave morphology, P-P interval, and P-R interval
GENERAL CONSIDERATIONS
This rhythm is benign
This rhythm and multifocal atrial tachycardia are similar except for heart rate
The other possible explanation is that there is significant respiratory sinus arrhythmia, with uncovering of latent foci of pacemaker activity
Usually, it is associated with underlying lung disease
In the elderly, it may be a manifestation of sick sinus syndrome
In the young and athletic heart, it may represent enhanced vagal tone
SYMPTOMS AND SIGNS
Usually causes no symptoms and is incidentally discovered
Occasional patient may feel skipped beats
PHYSICAL EXAM FINDINGS
Variable S 1
DIFFERENTIAL DIAGNOSIS
Multifocal atrial tachycardia (heart rate > 100 bpm)
Frequent premature atrial complexes and atrial bigeminy
LABORATORY TESTS
None specific
ELECTROCARDIOGRAPHY
ECG to document rhythm
CARDIOLOGY REFERRAL
Not required
MEDICATIONS
No specific treatment
Monitor and treat the underlying cause, such as sick sinus syndrome or lung disease
DIET AND ACTIVITY
No restrictions
General healthy lifestyle
Once a year if sinus node abnormality is suspected; otherwise when symptoms arise
COMPLICATIONS
May progress to sick sinus syndrome
This condition by itself is benign
PRACTICE GUIDELINES
Indications for pacemaker:
– If part of sick sinus syndrome
– If associated with documented symptomatic bradycardia
Sign in or create a free Access profile below to access even more exclusive content.
With an Access profile, you can save and manage favorites from your personal dashboard, complete case quizzes, review Q&A, and take these feature on the go with our Access app.
Pop-up div Successfully Displayed
This div only appears when the trigger link is hovered over. Otherwise it is hidden from view.
Please Wait

- Mobile Apps
- Journal Club
- Antibiotics
- Quick Critical Care
- Residency Directory
- Recent Changes
- About WikEM
- Getting Started
- Creating & Editing
- Needed Pages
- Editorial Levels
- Contribution Score
- Elective Guide
- Citing WikEM
- What links here
- Related changes
- Special pages
- Printable version
- Permanent link
- Page information
- Browse properties

- View source
- View history
- Create account

We need you! See something you could improve? Make an edit and help make WikEM better for everyone.
- Wandering atrial pacemaker
- 2 Clinical Features
- 3.1 Palpitations
- 4.2 Diagnosis
- 5 Management
- 6 Disposition
- 8 External Links
- 9 References
- Three or more ectopic foci within the atrial myocardium serve as the pacemaker
- Rate is less than 100bpm (in contrast to MAT )
- Is irregularly irregular therefore sometimes confused with atrial fibrillation and sinus arrhythmia
- Intrinsic cardiac or pulmonary disease
- Metabolic derangements
- Drug toxicity (including Digoxin )
Clinical Features
- Often seen in the extremes of age and in athletes
- Rarely causes symptoms
Differential Diagnosis
Palpitations.
- Narrow-complex tachycardias
- Wide-complex tachycardias
- Second Degree AV Block Type I (Wenckeback)
- Second Degree AV Block Type II
- Third Degree AV Block
- Premature atrial contraction
- Premature junctional contraction
- Premature ventricular contraction
- Sick sinus syndrome
- Acute coronary syndrome
- Cardiomyopathy
- Congenital heart disease
- Congestive heart failure (CHF)
- Mitral valve prolapse
- Pacemaker complication
- Pericarditis
- Myocarditis
- Valvular disease
- Panic attack
- Somatic Symptom Disorder
- Drugs of abuse (e.g. cocaine )
- Medications (e.g. digoxin , theophylline )
- Thyroid storm
- Pulmonary embolism
- Dehydration
- Pheochromocytoma

- ECG should show three distinct P wave morphologies with a ventricular rate <100bpm
- Rarely requires treatment
Disposition
- Outpatient management
- Multifocal atrial tachycardia
- Dysrhythmia
External Links
- Richard Cunningham
- fardis tavangary
- Ross Donaldson
- Privacy policy
- Disclaimers
Wandering Pacemaker
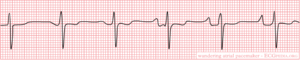
When several pacemakers are competing, p-waves with different origins and thus configurations occur. The rhythm is slightly different from beat to beat.
note If the heart rate increases to above 100bpm, it is called Multifocal Atrial Tachycardia . Possible causes are hypoxia, COPD and medication such as digoxin.
Navigation menu
Learn how UpToDate can help you.
Select the option that best describes you
- Medical Professional
- Resident, Fellow, or Student
- Hospital or Institution
- Group Practice
- Patient or Caregiver
- Find in topic
RELATED TOPICS
INTRODUCTION
This topic will present a broad review of the role of cardiac pacing in a variety of settings. The management of the specific disorders is discussed separately as is a description of the different types of pacemakers and pacing modes. (See "Sinus node dysfunction: Treatment" and "Third-degree (complete) atrioventricular block" and "Second-degree atrioventricular block: Mobitz type II" and "Modes of cardiac pacing: Nomenclature and selection" .)
GENERAL CONSIDERATIONS
● The association of symptoms with a bradyarrhythmia
● The location of the conduction abnormality

- Change Your Password
Search this Resource
Table of contents.
- Alternative/Holistic Medicine
- Anesthesia & Pain Management
- Animal Welfare
- Award Lectures
- Canine Myocardial Disease
- Cardiac Disease & Examination
- Cardiac Disease Diagnosis
- Arrhythmia Diagnosis & Management
- Pericardial Disease
- Interventional Cardiac Catheterization
- Feline Myocardial Diseases
- Valvular Heart Disease
- Clinical Immunology
- Clinical Pathology
- Critical Care
- Dermatology
- Diagnostic Imaging
- Emergency Medicine
- Endocrinology
- Exotics/Wildlife
- Fracture Surgery
- Gastrointestinal Surgery
- Joint Surgery
- NAVC How I Treat…
- Neurology/Surgery
- Oncologic Surgery
- One Health/Zoonotic
- Ophthalmology
- Pharmacology
- Physical Rehabilitation
- Reproduction
- Thoracic Surgery
- Urogenital Surgery
- Working Dog
Benefits and Limitations
The electrocardiogram (ECG or EKG) provides a graphic representation of the electrical depolarization and repolarization processes of the cardiac muscle, as "viewed" from the body surface. The amplitude of these electrical potential differences between various points on the body is measured in millivolts (mV) and their duration in seconds. The ECG can provide information on heart rate, rhythm, and intracardiac conduction; it may also reveal evidence of specific chamber enlargement, myocardial disease or ischemia, pericardial disease, certain electrolyte imbalances, and some drug toxicities. But note that although the ECG is a valuable part of the cardiac evaluation, it cannot determine if congestive heart failure is present, or (in itself) predict whether an animal will survive procedures requiring anesthesia, nor can it provide much information on the strength (or even presence) of cardiac contractions.
Sinus rhythm is the normal cardiac rhythm, described above. The P waves are positive in the caudal leads (II and aVF), the P-Q intervals are consistent and the R-R intervals occur regularly, with less than 10% variation in timing. Normally, the QRS complexes are narrow and upright in leads II and aVF; however, if an intraventricular conduction disturbance or ventricular enlargement pattern is present, they may be wide and abnormally shaped.
Sinus bradycardia is a rhythm that originates in the sinus node and is conducted normally but has too slow a rate, while sinus tachycardia also originates in the sinus node and is conducted normally but is too rapid.
Sinus arrhythmia is characterized by a cyclical slowing and speeding of the sinus rate, most commonly associated with respiration. The rate tends to increase on inspiration and decrease with expiration because of changes in vagal tone. Often, there is an accompanying change in P wave configuration (wandering pacemaker) with the P waves becoming taller and spiked during inspiration and flatter in expiration. Marked sinus arrhythmia occurs in some animals with chronic pulmonary disease. Sinus arrhythmia is a normal rhythm variation . It is commonly seen in dogs, but not often in the clinical setting in normal cats. However, cats frequently have sinus arrhythmia when relaxed or sleeping.

Sinus arrest is a cessation of sinus node activity lasting at least twice as long as the patient's longest expected R-R interval. The resulting pause in heart rate is interrupted by either an escape beat or resumption of sinus activity. Fainting or weakness may result during these pauses.
Conduction blocks in the major ventricular conduction system also disturb the normal activation process and result in altered QRS configurations. The portion of the ventricles served by the diseased bundle branch is activated late and slowly, resulting in widening of the QRS with the terminal forces oriented toward the area of delayed activation.
Rhythm Disturbances
Impulses originating from outside the sinus node are abnormal and create an arrhythmia (dysrhythmia). Abnormal or ectopic impulses are described based on their site of origin (atrial, junctional, supraventricular, ventricular). They are also characterized by timing , that is, whether they occur earlier than the next expected sinus impulse ( premature ) or whether they occur late ( escape ), as a rescue mechanism. Abnormal premature impulses (complexes) may occur singly or in multiples. Groups of three or more comprise an episode of tachycardia ; bouts of tachycardia may be brief (paroxysmal tachycardia) or quite prolonged (sustained tachycardia). A bigeminal pattern occurs when each normal QRS is followed by a premature complex; the origin of the premature complexes determines whether the rhythm is atrial or ventricular bigeminy.

Supraventricular (atrial, junctional) premature complexes originate above the AV node, in either the atrium or the AV junctional (near the AV node) area; however, since they are conducted through the ventricles in the normal manner, their QRS configuration is normal (unless an intraventricular conduction disturbance is also present). Atrial premature complexes are preceded by an abnormal P wave (either positive, negative or biphasic).
Ventricular premature complexes (VPCs or PVCs) originate below the AV node and do not activate the ventricles by the normal pathway; therefore, they have an abnormal ECG configuration. Ventricular ectopic complexes are also wider than the normal QRS complexes because of their slower conduction through ventricular muscle. When the configuration of VPCs or tachycardia in a patient is consistent, the complexes are described as being uniform or unifocal. When the VPCs occurring in an individual have differing configurations, they are said to be multiform. Increased electrical instability of the heart is thought to accompany multiform VPCs or tachycardia. Ventricular tachycardia defines a rapid series of VPCs (greater than 100 beats/minute in the dog, for example). The R-R interval is usually regular, although some variation is not uncommon. Sinus P waves may be seen superimposed on or between the ventricular complexes; they are unrelated to the VPCs because the AV node and/or ventricles are in the refractory period (physiologic AV dissociation).

Atrial fibrillation ("delirium cordis") is a common arrhythmia characterized by rapid, chaotic electrical activation of the atria. There are no P waves on the ECG; rather, the baseline usually shows irregular undulations (fibrillation waves). Since there is no organized electrical activity, meaningful atrial contraction is absent. The AV node, being constantly bombarded with these disorganized electrical impulses, conducts as many as possible to the ventricles. The (ventricular) heart rate is, therefore, determined by how many impulses the AV node can conduct. Atrial fibrillation results in an irregular heart rhythm, which is usually quite rapid. Most often, the QRS complexes appear normal in configuration, since the normal intraventricular conduction pathway is used. Atrial fibrillation tends to be a consequence of significant atrial disease and enlargement in small animals.

Atrio - ventricular (AV) conduction blocks may result from therapy with certain drugs, high vagal tone, and organic disease of the AV node and/or ventricular conduction system. AV blocks are also called "Heart Blocks."

Matthew W. Miller, DVM, MS, DACVIM (Cardiology) College of Veterinary Medicine and Biomedical Sciences Texas A&M University College Station, TX, USA

- January 2024 - Volume 15 Issue 1
- February 2024 - Volume 15 Issue 2
- March 2024 - Volume 15 Issue 3
- January 2023 - Volume 14 Issue 1
- February 2023 - Volume 14 Issue 2
- March 2023 - Volume 14 Issue 3
- April 2023 - Volume 14 Issue 4
- May 2023 - Volume 14 Issue 5
- June 2023 - Volume 14 Issue 6
- July 2023 - Volume 14 Issue 7
- August 2023 - Volume 14 Issue 8
- September 2023 - Volume 14 Issue 9
- October 2023 - Volume 14 Issue 10
- November 2023 - Volume 14 Issue 11
- December 2023 - Volume 14 Issue 12
- January 2022 - Volume 13 Issue 1
- February 2022 - Volume 13 Issue 2
- March 2022 - Volume 13 Issue 3
- April 2022 - Volume 13 Issue 4
- May 2022 - Volume 13 Issue 5
- June 2022 - Volume 13 Issue 6
- July 2022 - Volume 13 Issue 7
- August 2022 - Volume 13 Issue 8
- September 2022 - Volume 13 Issue 9
- October 2022 - Volume 13 Issue 10
- November 2022 - Volume 13 Issue 11
- December 2022 - Volume 13 Issue 12
- January 2021 - Volume 12 Issue 1
- HD Grid Supplement - Volume 12 Issue S1
- February 2021 - Volume 12 Issue 2
- March 2021 - Volume 12 Issue 3
- April 2021 - Volume 12 Issue 4
- May 2021 - Volume 12 Issue 5
- June 2021 - Volume 12 Issue 6
- July 2021 - Volume 12 Issue 7
- August 2021 - Volume 12 Issue 8
- September 2021 - Volume 12 Issue 9
- October 2021 - Volume 12 Issue 10
- November 2021 - Volume 12 Issue 11
- December 2021 - Volume 12 Issue 12
- January 2020 - Volume 11 Issue 1
- February 2020 - Volume 11 Issue 2
- March 2020 - Volume 11 Issue 3
- April 2020 - Volume 11 Issue 4
- May 2020 - Volume 11 Issue 5
- June 2020 - Volume 11 Issue 6
- July 2020 - Volume 11 Issue 7
- August 2020 - Volume 11 Issue 8
- September 2020 - Volume 11 Issue 9
- October 2020 - Volume 11 Issue 10
- November 2020 - Volume 11 Issue 11
- December 2020 - Volume 11 Issue 12
- January 2019 - Volume 10 Issue 1
- February 2019 - Volume 10 Issue 2
- March 2019 - Volume 10 Issue 3
- April 2019 - Volume 10 Issue 4
- May 2019 - Volume 10 Issue 5
- June 2019 - Volume 10 Issue 6
- July 2019 - Volume 10 Issue 7
- August 2019 - Volume 10 Issue 8
- September 2019 - Volume 10 Issue 9
- October 2019 - Volume 10 Issue 10
- November 2019 - Volume 10 Issue 11
- December 2019 - Volume 10 Issue 12
- January 2018 - Volume 9 Issue 1
- February 2018 - Volume 9 Issue 2
- March 2018 - Volume 9 Issue 3
- April 2018 - Volume 9 Issue 4
- May 2018 - Volume 9 Issue 5
- June 2018 - Volume 9 Issue 6
- July 2018 - Volume 9 Issue 7
- August 2018 - Volume 9 Issue 8
- September 2018 - Volume 9 Issue 9
- October 2018 - Volume 9 Issue 10
- November 2018 - Volume 9 Issue 11
- December 2018 - Volume 9 Issue 12
- January 2017 - Volume 8 Issue 1
- February 2017 - Volume 8 Issue 2
- March 2017 - Volume 8 Issue 3
- April 2017 - Volume 8 Issue 4
- May 2017 - Volume 8 Issue 5
- June 2017 - Volume 8 Issue 6
- July 2017 - Volume 8 Issue 7
- August 2017 - Volume 8 Issue 8
- September 2017 - Volume 8 Issue 9
- October 2017 - Volume 8 Issue 10
- November 2017 - Volume 8 Issue 11
- December 2017 - Volume 8 Issue 12
- January 2016 - Volume 7 Issue 1
- February 2016 - Volume 7 Issue 2
- March 2016 - Volume 7 Issue 3
- April 2016 - Volume 7 Issue 4
- May 2016 - Volume 7 Issue 5
- June 2016 - Volume 7 Issue 6
- July 2016 - Volume 7 Issue 7
- Special White Paper
- August 2016 - Volume 7 Issue 8
- September 2016 - Volume 7 Issue 9
- October 2016 - Volume 7 Issue 10
- November 2016 - Volume 7 Issue 11
- December 2016 - Volume 7 Issue 12
- January 2015 - Volume 6 Issue 1
- February 2015 - Volume 6 Issue 2
- March 2015 - Volume 6 Issue 3
- April 2015 - Volume 6 Issue 4
- May 2015 - Volume 6 Issue 5
- June 2015 - Volume 6 Issue 6
- July 2015 - Volume 6 Issue 7
- August 2015 - Volume 6 Issue 8
- September 2015 - Volume 6 Issue 9
- October 2015 - Volume 6 Issue 10
- November 2015 - Volume 6 Issue 11
- December 2015 - Volume 6 Issue 12
- Fellows Edition Supplement
- Author Guidelines
- Submit Manuscript
- Editorial Board
- About JICRM

A Review of Temporary Permanent Pacemakers and a Comparison with Conventional Temporary Pacemakers
DOI: 10.19102/icrm.2019.100506
KEITH SUAREZ, MD 1 and JAVIER E. BANCHS, MD , FACC , FHRS 1
1 Section of Electrophysiology & Pacing, Division of Cardiology, Department of Medicine, Baylor Scott & White Temple Memorial Hospital, Baylor Scott & White Health, Dallas, TX, USA
ABSTRACT. Temporary cardiac pacing is commonly used in patients with life-threatening bradycardia and serves as a bridge to implantation of a permanent pacemaker (PPM). For years, passive fixation leads have been used for this purpose, offering the advantage of that they can be placed at bedside. The downside, however, is that patients must remain on telemetry and bed rest until lead removal due to the risk of displacement and failure to capture. Even then, the latter cannot always be prevented. Temporary cardiac pacing with passive fixation leads has also been related to a higher incidence of infection and venous thrombosis, delayed recovery, and increased length of stay. Thus, over the last couple of decades, pacemaker leads with an active fixation mechanism have become increasingly used. This is known as a temporary PPM (TPPM) approach, which carries a very low risk of lead dislodgement and allows patients to ambulate, among other advantages. Here, we performed a review of the literature on the use of TPPMs and their advantages over temporary pacemakers with passive fixation leads and in order to evaluate the advantages and disadvantages of active and passive fixation leads in temporary cardiac pacing. Most articles found were case reports and case series, with few prospective studies. We excluded documents such as editorials and image case reports that provided little to no useful information for the final analysis. The literature search was performed in PubMed, Google Scholar, and other databases and articles written in English and Spanish were considered. Articles were screened up to January 2017. The search keywords used were “temporary permanent pacemaker,” “external permanent pacemaker,” “active fixation lead,” “explantable pacemaker,” “hybrid pacing,” “temporary permanent generator,” “prolonged temporary transvenous pacing,” and “semipermanent pacemaker.” A total of 24 studies with 770 patients were ultimately included in our review. The age group was primarily above the sixth decade of life, with the exception of one that included pediatric patients. Indications for pacing included device infection, sick sinus syndrome, atrioventricular block, ventricular tachycardia, and bradyarrhythmias associated with systemic illness. The duration of TPPM usage varied from a few days up to 336 days. A total of 18 (2.3%) TPPM-related infections were reported, in which the duration of TPPM use was less than 30 days in at least 15 patients. Loss of capture was documented in only eight patients (1.0%). Complication rates varied from 0% to 30%, with the highest event rates being present in studies that used femoral venous access. In conclusion, although no high-quality studies were identified in our literature search, we found the data retrieved suggest the association of overall favorable outcomes with the use of TPPMs. Device placement and removal typically involve a simple procedure, although fluoroscopy, usually applied in the cardiac catheterization laboratory, is necessary for implantation, which could represent an additional risk in a patient who is already hemodynamically unstable. When possible, a screw-in-lead pacemaker should be used for temporary pacing.
KEYWORDS. Active fixation lead, cardiac pacing, pacemaker, passive fixation lead.
The authors report no conflicts of interest for the published content. Manuscript received September 10, 2018. Final version accepted November 28, 2018. Address correspondence to: Keith Suarez, MD, 5227 West Adams Avenue, Apt 122, Temple, TX 76502, USA. Email: [email protected] .
Introduction
Initial descriptions of pulsed electrical stimulation to the heart can be attributed to J. A. McWilliam in the late 19th century. 1 Subsequently, the first pacemaker device was built by the American physiologist Albert Hyman in 1932. In 1952, Drs. John Callaghan and Wilfred Bigelow and engineer Jack Hopps developed a bipolar catheter able to provide endocardial stimulation. Zoll Medical Corporation (Chelmsford, MA, USA) later developed an external pacing system with cutaneous electrodes. In 1959, Seymour Furman and John Schwedel were able to provide endocardial stimulation by utilizing a lead inserted through the internal jugular vein. The first attempts to employ an implantable pacemaker were performed in Sweden in 1958. 1 Most publications only refer to Furman when addressing the history of pacemakers.
Pacemakers function by way of electrically stimulating the myocardium to increase the heart rate for the treatment of bradyarrhythmias, or, in specific cases, to prevent or treat a tachyarrhythmia (eg, QT-shortening in long QT syndrome, circuit entraining in atrial flutter and ventricular tachycardia). 2 , 3 Their use can be either temporary or permanent, depending on the indication. Temporary pacing is preferred in the setting of an emergency, since it is more readily available. Temporary pacing can serve as a bridge to a permanent device or recovery, although the time to recovery can be lengthy in conditions such as Guillain–Barré disease, Lyme disease, and tetanus. 4 , 5
The placement of both permanent pacemakers (PPMs) and implantable cardioverter-defibrillators in the United States increased from 1997 to 2004 by 19% and 60%, respectively. 6 Most patients who receive these devices are elderly and, as this age group continues to grow, the number of devices implanted will likely increase—as will the rate of complications. An analysis from 1997 to 2004 in the United States population reported that 70% of patients who received a device were older than 65 years of age. 7
Patients with a PPM who develop a pocket infection, secondary bacteremia, or endocarditis have a class I indication for complete removal of the device due to the high recurrence of infection associated with antibiotic therapy only. 7 , 8 However, if the patient happens to be pacemaker-dependent, they would require temporary pacing in such a situation until the infection has been treated. Prior studies have suggested that the incidence of cardiac implantable electronic device (CIED) infections is 1% to 7%, with a 2.8-fold increase for PPMs and a six-fold increase for ICDs occurring between 1996 and 2003. 7
The leads more commonly used for temporary pacing are leads with no or passive fixation. Some have tines at the distal end and are positioned so that they can hold onto myocardial trabeculations. This feature heightens the risk of lead dislodgement when compared with the composition of an active fixation lead, which is also known as a temporary PPM (TPPM) lead ( Figure 1 ) . 9 Some risk factors for dislodgement are modifiable (eg, noncooperative patient, 10 inadvertent movement of the limbs, site of venous access, inadequate positioning of the lead), while others are more difficult to troubleshoot (eg, ventricular contraction, anatomy of the right heart and great veins, nonfixation nature of the lead). The reported incidence of dislodgement varies among publications (10%–60%) and is consistently higher with passive fixation leads.
In a review article, the most common complications reported with passive fixation leads were failure of venous access (15%), failure to place a lead (10%), and sepsis (9%). 11 Hyman et al. studied 1,022 patients at the Mayo Clinic who required conventional temporary pacing. 9 Lead dislodgement occurred in 17.9% of patients and was the most common complication observed. The overall mortality rate was reported to be 17.6% and it was not clear as to whether or not this was a consequence of the temporary pacing itself or other factors. Another single-center retrospective study with 530 cases described a dislodgement rate of 9%, with 99% of venous access occurring through the femoral route. 10 A total of 34 patients died, with three deaths being attributed to complications associated with the pacemaker (0.6% of all cases; 8.8% of all deaths).
The occurrence of deep vein thrombosis (DVT) and pulmonary embolism correlates primarily with the route of venous access. Nolewajka et al. studied venograms and autopsies that were completed in patients with femoral venous pacemakers. 12 , 13 The incidences of femoral DVT and pulmonary embolism were 34% and 50%, respectively. Some physicians anticoagulate all of their patients, which thus adds bleeding as a potential other complication. 10 Interestingly, a separate report of 113 patients with temporary pacemakers showed that only femoral pacemakers caused pulmonary embolism as compared with brachial ones. 14 This route has become much less popular over time, and a shift toward utilizing the right internal jugular vein route instead was even highlighted at the time of Hyman et al.’s study. 9 Local infection and sepsis are also known to occur more frequently in conjunction with femoral venous access. 14
Other strategies were considered in previous decades before active fixation leads came into play. Most of these were applied in patients with a history of device infection who required temporary pacing during antibiotic treatment. In a study from 1971, four patients with infected devices were managed by opening the pocket, performing debridement, and reclosing the pocket right after. 15 In 1984, investigators evaluated six patients who presented with pacemaker erosion. 16 They were managed by way of exteriorizing the device and attaching it instead to the patient’s neck. Once antibiotics were completed, the infected device was replaced by a new one. Another case series studied a similar protocol and reported good outcomes as well. 17 One study did reveal a higher recurrence rate of infection of 77% if only the generator was removed versus a rate of 8% if the leads were extracted too. 18 The use of antibiotic therapy added to wound care with no device removal resulted in poor infection resolution, constituting the reason for why this approach is not recommend at the present time. 19
In 1973, researchers employed a pacing method known as semipermanent pacing. 20 In this approach, they placed a lead through the cephalic vein and connected it to a temporary pacemaker. If, after a variable period of time, this lead remained in a stable position, it was then connected to a PPM. In 1984, the use of external PPMs in DDD mode for temporary pacing was reported in 13 patients for the treatment of bradyarrhythmias and overdrive pacing. 21 Eight patients benefited from treatment with and nine were ambulatory while using this device. Other authors have reproduced these findings. 22 In postcardiac surgery patients, epicardial leads can be connected to an exteriorized extension and a temporary pacemaker. These leads can be later used for permanent pacing if necessary. 23 Furthermore, in addition, epicardial leads are located outside of the intravascular space and have a lower risk of bloodstream infections. One study found TPPM patients to have a longer hospital stay than those with epicardial leads, although the reason for this finding was not clear. 24
In this study, we aimed to determine the advantages and disadvantages of employing TPPMs with active fixation leads versus standard temporary pacing. Specifically, we evaluated the length of hospital stay in terms of number of days, rate of secondary infections and venous thrombosis, incidence of loss of capture, overall rate of complications, costs, and deaths.
Search strategy
An online search of the PubMed, Google Scholar, OVID, and EBSCO databases was performed. We searched for articles written in either English and/or Spanish and identified all relevant articles available until January 2017. The search words applied were “temporary permanent pacemaker,” “external permanent pacemaker ,” “ active fixation,” “explantable pacemaker ,” “ hybrid pacing,” “temporary permanent generator,” “prolonged temporary transvenous pacing,” and “ semipermanent pacemaker.”
No systematic reviews, meta-analyses, or randomized control trials were found. Most articles included were full-text versions and included case reports, case series, and prospective observational studies. We excluded articles with insufficient information available as well as review articles. If an abstract was deemed to have sufficient information, it was included. One study evaluating the new Tempo Lead (BioTrace Medical, San Carlo, CA, USA) presented at the 2016 Transcatheter Cardiovascular Therapeutics meeting was also excluded. 25
Variables included in our analysis were age, number of patients, follow-up time, duration of temporary pacing, single-group versus comparison-group study, rate of secondary infections, rate of lead dislodgement, single-chamber versus dual-chamber pacing, pacing threshold, death, average time to discharge from implantation of the temporary lead, costs, overall complications, and early ambulation. Relevant data were extracted from the articles and then represented in an Excel spreadsheet (Microsoft Corp., Redmond, WA, USA) to later generate tables ( Tables 1 – 4 ) . Information on certain variables was missing in some studies.
Thirty-one relevant articles were found. Of these, seven were excluded because they were editorials, review articles, or had insufficient information. This left us with 24 articles. Six studies did not have a clear design method; a total of five were case reports; and, among the case series identified, three were prospective, seven were retrospective, and one combined a retrospective control group with a validation prospective group. The prospective studies were not randomized. Four studies reported having conflicts of interest and another four stated having none.
Martin et al. appeared to be the first to publish a report on the use of TPPMs. 26 Their publication was available as a supplement. No lead dislodgements were reported, and patients were able to ambulate quickly without a need for telemetry. Two deaths occurred, although neither happened as a complication of the pacemaker implant.
All studies were single-center. Eight reported the use of atrial pacing with active fixation leads. Limited data were available about the use of temporary dual-chamber pacing and tunneled leads. One study was not clear regarding the duration of temporary pacing. Most used the internal jugular vein for access and, as second option, the subclavian vein. Seven studies reported on ambulation, while only two quantified the number of patients who did ambulate. Other studies only mentioned whether patients were allowed to ambulate or not. Regarding complications, only one publication did not report on the rate of TPPM infection, while two did not report on loss of capture. Little was reported on secondary deep venous thrombosis. The overall complication rate (excluding death) ranged between 0% and 30%. No complications occurred in 12 studies, while seven studies reported the rate of complications to be between 3% and 10%. Pecha et al. reported no complications after a mean follow-up time of 21.2 months including recurrent infection, lead dislodgement, or death. 27 Zei et al. reported a case series of 62 patients with no documentation of lead dislodgements, device infections, or perforations after a median duration of temporary pacing for 7.5 days. 28 Most complications were observed in three studies in which only femoral access was used; De Cock et al. found rates of 26% and 30%, respectively, 29 , 30 while Garcia et al. noted a rate of 17%. 31
Among the 24 articles, a total of 770 patients were studied. Most patients were of an advanced age. The study from Pinto et al. was the only one that included pediatric patients. 32 Eighteen studies reported on gender distribution, with a total of 253 males (64.9%) and 137 females (35.1%) having a TPPM placed. Indications for the use of TPPM included device infection, bradyarrhythmias, ventricular tachycardia, and transcatheter aortic valve replacement ( Table 1 ) . Device infection was cited as the most common indication. Kornberger et al. reported TPPM use for this indication in 70% of their patients, while, in Rastan et al.’s study, such was the indication in all of 10 patients. 33 , 34 When reported, the duration of TPPM was widely variable and most often ranged between 10 days and 20 days. The lengthiest duration of TPPM was 36 months, as reported by Pecha et al., 27 while the shortest was one day, per Braun et al. 35 ( Table 2 ) .
Three studies had a control group with passive fixation leads, and one study compared TPPMs with epicardial leads. After excluding patients in the control groups who were treated with passive fixation, the total number of patients with TPPMs was 708. We then calculated the total percentage of patients with TPPMs who developed an infection to be 2.5%. For loss of capture, we found eight patients in the TPPM group were affected, which corresponds to 1.7% of the total number of patients ( Table 3 ) . Among individual studies, we highlight De Cock, who demonstrated a lead dislocation of 5% in TPPM patients versus that of 33% in passive lead pacing patients and total adverse events rates of 30.6% and 58.1%, respectively. 29 This difference was evident after 5.8 days ± 2.9 days of follow-up. 29 Chihrin et al. only reported one dislodgement out of 20 patients. 36 Amraoui et al. saw no dislodgements in 80 patients treated with TPPM placement. 24
Of the 24 articles reviewed, a total of 18 infections of the TPPM system were reported ( Table 3 ) . This number could have been even smaller if the venous access in De Cock et al.’s studies would have been subclavian or jugular rather than femoral. 24 , 28 Furthermore, these two investigations reported 11 of the 18 infections that we identified in our literature search. In Kornberger et al.’s study, three TPPMs were removed due to signs of systemic infection, although it was never proven that TPPM usage was the culprit. 37 In Kawata et al.’s study, the only patient known to have a complication had a lead vegetation and their lead was replaced. 38
Thirteen studies reported pacing thresholds. All were measured below 1.5 V except in a study by De Cock et al. that reported a range of 1.36 V ± 0.65 V. 30 One study reported a lower pacing threshold in the conventional pacing group, although the difference did not appear to be clinically significant. 29 It improved in the TPPM group after a 24-hour period. Braun et al. reported a median pacing threshold of 0.6 V in the active fixation lead group, which was minimally lower when compared to that in the passive lead group. 35 Additionally, six studies reported on the average time to discharge. The mean time varied from 11.3 days to 30.7 days. Early discharge was more likely to be achieved in patients with less severe device infections and bradyarrhythmias.
All studies reported a death rate ( Table 3 ) . Specifically, there were 84 deaths reported, but only six of these were deemed by the authors to be attributed in some fashion to the pacemaker itself. Most of the deaths were a consequence of either multiorgan dysfunction related to cardiogenic shock, overwhelming sepsis, or refractory ventricular arrhythmias. Only two studies assessed costs. Chihrin et al. found that, in the first 18 hours of use, the costs of TPPM placement were higher due to the price of the active fixation lead. 34 The price of the pacemaker generator was not included, as it is reusable. After this period, they concluded a TPPM would save $456 per 24-hour interval in comparison with passive fixation leads. Lever et al. also reported reduced costs with TPPM placement. 39 Obviating the need to use a bed in the cardiac care unit likely reduces costs related to the provision of an advanced level of care.
All studies used VVI pacing except for one that used VDD, 40 and eight described the use of atrial pacing. Pang et al. reported on two patients who were paced in VVI mode and who became hypotensive due to atrioventricular dyssynchrony. 41 After placement of an atrial lead, they improved clinically. Orsbourn et al. also reported on the use of dual-chamber pacing in seven of the 23 patients they studied. 42 Lepillier et al. followed eight patients with complete heart block and heart failure who had temporary dual-chamber pacemakers placed and observed an improvement in heart failure symptoms and brain natriuretic peptide levels. 40 Level of activity was reported in 10 studies ( Table 4 ) . Some patients had to remain in bed despite TPPM placement because of other comorbidities. 31 , 35 , 43
In two studies by De Cock et al., ambulation was reported as occurring in 75% and 73%. 29 , 30 Spontaneous loss of capture was not documented. One patient removed his pacing lead secondary to delirium. Garcia et al. prospectively assessed 47 patients who had received a femoral TPPM 31 and classified them into the categories of high, moderate, and low mobility. Only three out of the 12 patients in the low-mobility group had a DVT, while such was not documented at all in those with medium or high mobility. They compared their findings with those from an older study with an incidence of 25% to 39% of asymptomatic DVT achieved when using passive fixation leads. 44 De Cock et al. also reported that only one out of 42 patients developed DVT. 29 All of these patients were being anticoagulated with intravenous heparin, which likely confounded the outcome.
Two of the reviewed studies had a group with passive fixation leads for comparison with the TPPM group. 30 , 35 Braun et al. in 2006 compared 23 patients treated with TPPM placement and 26 treated with a passive fixation lead. Infection was not reported in either group. There were 24 “loss-of-capture” events in the passive fixation group versus one in the active fixation group (p < 0.01). Three patients in the first group required resuscitation on more than one occasion, which prompted pacing with a TPPM.
Thanks to a screw-in mechanism, the active fixation lead provides greater stability and reliable pacing. 9 , 38 , 45 Intermittent loss of capture during temporary pacing is a relatively common cause of intensive care unit (ICU) emergencies in part because prolonged pacing can suppress ventricular escape and precipitate asystole if loss of capture occurs. 46 The added results of our review show a 1.7% dislodgement rate for TPPM. This benefit was noticeable even when TPPM was used for months. 38 , 45 The value of this finding remains in patients who might require temporary pacing for long periods of time. 32 , 33
Passive leads are often used in patients who are hemodynamically unstable and who cannot be transported to a procedure room. The parameters used in assessing proper placement are length of lead inserted, telemetry monitoring that confirms ventricular capture, and chest X-ray. 37 Screw-in leads ideally require transferring the patient to the catheterization laboratory for placement under fluoroscopy to ensure that the screw is deployed in the proper position. The dislodgement rate when using passive fixation leads has been reported at 17% with femoral leads after 4.8 days of follow-up in a series of 100 patients, 47 while other studies have suggested it to be between 10% and 30%. 48 , 49
Pacing thresholds when using TPPM have been reported to be less than 1 V in most studies. 26 , 38 , 42 , 43 , 50 Similar to the placement of permanent pacemakers or passive fixation leads, a low capture threshold is one of the parameters used to determine proper placement of the pacing electrode. It is recommended that pacing and sensing be programmed in a bipolar fashion, since the pacemaker generator is externalized. 50
The use of temporary pacing allows for the safe removal of an infected device, particularly in patients who are pacemaker-dependent. 51 After the infected device has been explanted, there needs to be a delay for implanting a new device starting from the first set of negative blood cultures, and this period of time is subjected to the presence of valvular endocarditis and extracardiac bacterial seeding. 52 Although small studies have shown good outcomes with the removal of an infected device and simultaneous placement of a new one, the availability of reliable temporary pacing using TPPMs does not justify managing patients in such a manner. In one study, Nandyala and Parsonet followed 68 patients with CIEDs and did not use TPPMs prior to extraction, instead implanting a new device at the contralateral site simultaneously. After a follow-up of more than one year, no recurrent infections were found. 53 Another retrospective review of 15 patients with same-day device implantation after lead extraction showed no recurrence of infection after a median follow-up of 44 months. 54 Simultaneous lead extraction and implantation of epicardial leads has also been reported in conjunction with good long-term outcomes, 55 with an overall complication rate similar to that of the transvenous route.
Concurrent infection of the temporary pacemaker can occur and, here, TPPMs appear to become infected less often than passive fixation leads. Most of the TPPM infections that we found were reported in research by De Cock et al., where transvenous femoral access was used routinely. 29 , 30 It has been well-described that there is an increased risk of infection from femoral venous lines, with the lowest being subclavian. 56 Among the reasons for why TPPMs may have a lower incidence of infection, one could consider the reduced manipulation of the lead, since loss of capture is infrequent and the entry site through the skin is smaller because a sheath does not have to be left in place, therefore minimizing bacteria seeding into the bloodstream. 36 , 50 The presence of comorbidities and the duration of pacing were similar when active and passive fixation lead cases were compared. 30 , 35
In one center, all TPPMs were placed with tunneled leads, with no report of secondary infections. 42 At this time, due to the low rate of infection associated with TPPMs, it is difficult to recommend the routine use of tunneled leads. Such may be considered in patients who are expected to use TPPMs for a very long period of time or who have other risk factors.
TPPMs are routinely placed contralaterally to the site where the permanent pacemaker is wanted. The right internal jugular vein is often approached in order to protect the subclavian veins that are generally used for permanent pacing. 24 Pneumothorax risk is low with internal jugular access guided by ultrasound, while the same risk during subclavian access can be minimized with ultrasound and fluoroscopic guidance. 57
It is still debatable as to whether the same site where the infected device was can be used for placement of a TPPM. 56 Some authors have explored placement of a temporary pacemaker through the same site where the infected pacemaker was, with the advantage of the new permanent device being located far from where the prior infection was found. 28 , 39 , 58 A potential disadvantage of this approach could be an increased risk for infection of the TPPM itself.
The procedure to place a TPPM is similar to that of a permanent pacemaker, with the exception of that a subcutaneous pocket is not needed. 6 Preparation and aseptic techniques are similar to those of placing a central venous catheter. 59 The anatomical landmark used when approaching the internal jugular vein is the angle between the two heads of the sternocleidomastoid muscle. Ultrasound will show the internal jugular vein and the common carotid artery, with the former being much more compressible. With ultrasound, we also can see the needle in real time as it advances through tissue. Once access is obtained, a J-shaped guidewire is advanced and a peelable sheath is threaded through it. Under fluoroscopic guidance, a pacemaker lead with a preformed stylet inside is advanced into the right ventricle and the screw is deployed either in the apex or the septum. Testing is done to ensure appropriate sensing, impedance, and capture thresholds. Once done, the sheath is peeled away and the lead is secured to the skin through the suture sleeve. The proximal end of the lead is inserted in the can and screwed, and the latter is finally attached to the patient’s skin with sutures and/or adhesives.
Some studies have addressed the use of temporal dual-chamber pacing. 40 – 42 This seems to be of the utmost importance in the setting of critical illness and known heart disease, where maintaining atrioventricular synchrony and optimal cardiac output becomes significant. Right ventricular pacing can cause atrioventricular dissociation leading to pacemaker syndrome as well as interventricular dyssynchrony with reduction of the left ventricular systolic function. 24 , 38 Dual pacing can also be achieved with a balloon-tipped single lead that includes noncontact atrial dipoles and which can perform overlapping biphasic impulse stimulation. 60 A caveat to routinely placing two leads instead of one is the potential for an increased risk of infection and thrombosis. It would be prudent to pace both the atrium and ventricle only when a significant hemodynamic benefit is expected.
Patients can be discharged from the hospital while still using a TPPM 38 , 45 , 56 and ambulation can often be resumed quickly. 30 , 36 , 39 , 45 This is not so in the case of passive fixation leads, which require a patient to be on bed rest and telemetry for 24 hours per day. The disadvantages of remaining on bed rest for long periods of time are well-described and include a risk for DVT, deconditioning, atelectasis, and increased hospital stay, among others. This becomes more important in patients who require prolonged temporary pacing such as those with CIED-related endocarditis. Ambulation in these patients is also promoted by the smaller size of the resterilized generator. 61
Loss of capture can still occur with active fixation leads, such as when a patient moves abruptly or during a lead extraction procedure. 24 Unintended dislodgement of the temporary lead could be prevented by positioning it at a certain distance from the leads to be extracted. 51
Most of the deaths documented were related to patient comorbidities. As an example, one study revealed that death was more frequent in patients who had a TPPM placed for an indication that was one other than infection of a CIED. 24 This is likely the case because most CIED infections are limited to the pocket site. In another example, Noble et al. reported the use of TPPM in 20 patients who had undergone transaortic valve replacement, a population that is expected to have a better outcome than those hospitalized in the ICU. 62 Here, there were only two deaths that occurred and none of these were secondary to the device itself. On the other hand, Dawood et al. reported a 29.6% mortality rate from etiologies that included ventricular fibrillation, respiratory failure, non-ST-segment-elevation myocardial infarction, abdominal aortic aneurysm rupture, stroke, and subdural hematoma. 63
Despite the fact that TPPMs were used for prolonged periods of time, such still was superior in terms of overall complication rates to conventional temporary pacemakers, 24 , 36 which have been reported to have rates as high as 30%. 9 , 14 , 64
The duration of hospital stay was rather prolonged with TPPM usage, likely from the underlying comorbidities. 24 If there was no other indication to continue being in the hospital, patients with TPPMs were usually able to leave for home or a nursing facility. This was not possible in those with passive fixation leads, since the indication for pacing had to be reversed to remove the temporary pacemaker or the patient need undergo placement of a permanent device. The shortest hospital stay was reported by Noble et al. (mean: 11.3 ± 4.7 days), while the longest was noted by Kornberger et al. (mean: 30.7 ± 23.8 days). 37 , 62
Few studies reported on the use of TPPMs that also were defibrillators. At present, it is difficult to justify this approach when wearable cardioverter-defibrillators are available, although it is common to learn that patients do not wear them consistently because of discomfort. Cooper et al. reported the case of a patient with an infected device who had multiple episodes of sustained ventricular tachycardia. 43 The external device used was a pacemaker and a defibrillator that allowed for the termination of these episodes with antitachycardia pacing with the avoidance of defibrillation.
Costs may be significantly reduced using active fixation leads. Only one publication at this time appears to have specifically addressed this question. 36 The reduction in costs was mainly determined by the reduced length of stay in the cardiac care unit and by obviating the use of telemetry. One problem with this study, however, is that it involved mainly patients with sleep apnea who volunteered to have a TPPM implanted and who would not have any other indication to stay in the ICU. If more ill patients were included, then a clear cost benefit may have not been as evident. Lever et al. also concluded that TPPM placement is associated with less costs. 39
The duration of TPPM use was variable, with some cases being as long as months and with a good safety margin. Certainly, the reliability of the active fixation mechanism allows for application for such extended periods. This can be justified in patients who remain critically ill who require a permanent pacemaker and who are at a high risk of complications if transported to a procedure room. In some uncommon situations, patients experienced a recovery of their conduction abnormalities after a lengthy hospital stay. 36 , 46 , 49 It may be wise to use a TPPM for as brief a period as possible in patients who have prosthetic material in their bodies due to the potential of bacterial seeding. These patients include those who undergo transcatheter aortic valve replacement, a population in which TPPMs are used frequently. 65
Conclusions
TPPMs constitute a safe modality for temporary pacing. The associated fixation mechanism and fairly easy placement make this type of device a superior option over conventional temporary pacing. We recommend it should be used as first-line and that passive fixation leads be limited to use in patients who are not stable enough to be transferred to a room with fluoroscopy.
However, it is important to note that most studies considered herein involved a small sample size and were single-center. Many did not report the time of follow-up. Designs were heterogeneous, hindering their comparison. Follow-up was reported in only nine studies. There was a comparison group with passive fixation leads in only two studies; thus, most authors compared their data to historical references. With the information available, we were unable to separate the critically ill from the noncritically ill individuals so as to establish the mortality rate for each.
Additionally, Chihrin et al. were the only authors to compare costs with nonactive fixation leads. Some studies assessed ambulation after TPPM placement, with four studies quantifying the number of patients who ambulated. Two studies assessed the presence of DVT with a large bias, since these used femoral venous access, and the majority of patients were anticoagulated with heparin. Few studies explored the use of dual-chamber and atrial pacing, knowing the potential hemodynamic benefits of maintaining atrioventricular synchrony.
- Ward C, Henderson S, Metcalfe N. A short history on pacemakers. Int J Cardiol. 2013;169(4):244–248. [CrossRef] [PubMed]
- Vedantham V, Badhwar N. Current indications for temporary and permanent cardiac pacing. In: Electrophysiological Disorders of the Heart. 2 nd ed. Vol. 1, Philadelphia, PA: Elsevier; 2012:429–439.
- Miyoshi F, Tanno K, Kobayashi Y. Suppression of torsades de pointes by biventricular pacing in a patient with long QT syndrome. Pacing Clin Electrophysiol. 2013;36(3):E67–E69. [CrossRef] [PubMed]
- Millo J, Culshaw M, Alp N, Salmon J. Semipermanent cardiac pacing in severe tetanus. Br J Anaesth. 2002;88(6):882. [PubMed]
- Kordouni M, Jibrini M, Siddiqui M. Long-term transvenous temporary pacing with active fixation bipolar lead in the management of severe autonomic dysfunction in Miller-Fisher syndrome: a case report. Int J Cardiol. 2007;117(1):e10–e12. [CrossRef] [PubMed]
- Baddour LM, Epstein AE, Erickson CC, et al. A summary of the update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. J Am Dent Assoc. 2011;142(2):159–165. [CrossRef] [PubMed]
- Mulpuru S, Pretorius V, Birgersgotter-Green U. Device infections: management and indications for lead extractions. Circulation. 2013;128(9):1031–1038. [CrossRef] [PubMed]
- Schwartz IS, Pervez N. Bacterial endocarditis associated with a permanent transvenous cardiac pacemaker. JAMA. 1971;218(5):736–737. [CrossRef] [PubMed]
- Hynes J, Holmes D, Harrison C. Five-year experience with temporary pacemaker therapy in the coronary care unit. Mayo Clin Proc. 1983;58(2):122–126. [PubMed]
- McCann P. A review of temporary cardiac pacing wires. Indian Pacing Electrophysiol J. 2006;7(1):40–49. [PubMed]
- López Ayerbe J, Villuendas Sabaté R, García García C, et al. [Temporary pacemakers: current use and complications.] Rev Esp Cardiol. 2004;57(11):1045–1052. Article in Spanish. [PubMed]
- Nolewajka AJ, Goddard MD, Brown TC. Temporary transvenous pacing and femoral vein thrombosis. Circulation. 1980;62(3):646–650. [PubMed]
- Pandian NG, Kosowsky BD, Gurewich V. Transfemoral temporary pacing and deep vein thrombosis. Am Heart J. 1980;100(6 Pt 1):847–851. [CrossRef] [PubMed]
- Austin JL, Preis LK, Crampton RS, Beller GA, Martin RP. Analysis of pacemaker malfunction and complications of temporary pacing in the coronary care unit. Am J Cardiol. 1982;49(2):301–306. [CrossRef] [PubMed]
- Bonchek LI. New methods in the management of extruded and infected cardiac pacemakers. Ann Surg. 1972;176(5):686–689. [PubMed]
- Tyndall EC, Goodwin SD. Temporary use of an eroded bipolar pacemaker system. Ann Surg. 1984;50(8):446–449. [PubMed]
- Antinori CH, Villanuea DT, Pierucci L Jr, Manuele VJ, Kuchler JA. A new approach to the management of infected pacemakers. Clin Cardiol. 1994;17(1):38–40. [CrossRef] [PubMed]
- Harjula A, Jarvinen A, Virtanen KS, Mattila S. Pacemaker infections—treatment with total or partial pacemaker system removal. Thorac Cardiovasc Surg. 1985;33(4):218–220. [CrossRef] [PubMed]
- Furman RW, Hiller AJ, Playforth RH, Bryant LR, Trinkle JK. Infected permanent cardiac pacemaker. Management without removal. Ann Thorac Surg. 1972;14(1):55–58. [PubMed]
- Meibom J, Haghfelt T, Leth A. Semipermanent implantation of cardiac pacing electrodes by the use of the cephalic veins. Dan Med Bull. 1973;20(5):132–136. [PubMed]
- Littleford PO, Schwartz KM, Pepine CJ. A temporary external DDD pacing unit. Am J Cardiol. 1984;53(8):1041–1043. [PubMed]
- Ferguson Tb Jr, Cox JL. Temporary external DDD pacing after cardiac operations. Ann Thorac Surg. 1991;51(5):723–732. [CrossRef] [PubMed]
- Nathan M, Yahr W, Greenberger J. Temporary-permanent pacemaker lead for cardiac surgery. J Thorac Cardiovasc Surg. 1972;64(6):957–958. [PubMed]
- Amraoui S, Sohal M, Li A, et al. Comparison of delayed transvenous reimplantation and immediate surgical epicardial approach in pacing-dependent patients undergoing extraction of infected permanent pacemakers. Heart Rhythm. 2015;12(6):1209–1215. [CrossRef] [PubMed]
- Webster M, Pasupati S, Lever N, Stiles M. First-in-human experience with a novel active fixation temporary pacing lead: safety and efficacy of the Tempo Lead. J Am Coll Cardiol. 2016;68(18):B339–B340.
- Martin D, Vergara I, Greenfield R, Sorrentino R. Prolonged temporary transvenous pacing using permanent active-fixation leads. Circulation. 1999;100(18):I-786.
- Pecha S, Aydin MA, Yildirim Y, et al. Transcutaneous lead implantation connected to an externalized pacemaker in patients with implantable cardiac defibrillator/pacemaker infection and pacemaker dependency. Europace. 2013;15(8):1205–1209. [PubMed]
- Zei PC, Eckart RE, Epstein LM. Modified temporary cardiac pacing using intravenous active fixation leads and external resterilized pulse generators. J Am Coll Cardiol. 2006;47(7):1487–1489. [CrossRef] [PubMed]
- De Cock C, Van Campen C, Veld J, Visser C. Utility and safety of prolonged temporary transvenous pacing using an active-fixation lead: comparison with a conventional lead. Pacing Clin Electrophysiol. 2003;26(5):1245–1248. [CrossRef] [PubMed]
- De Cock CC, Van Campen LC, Visser CA. Usefulness of a new active-fixation lead in transvenous temporary pacing from the femoral approach. Pacing Clin Electrophysiol. 2003;26(4 Pt 1):849–852. [PubMed]
- Garcia-Guerrero JJ, Castaneda JF, Quero DL, et al. Lower incidence of venous thrombosis with temporary active-fixation lead implantation in mobile patients. Europace. 2010;12(11):1604–1607. [CrossRef] [PubMed]
- Pinto N, Jones TK, Dyamenahalli U, Shah MJ. Temporary transvenous pacing with an active fixation bipolar lead in children. Pacing Clin Electrophysiol. 2003;26(7 Pt 1):1519–1522. [PubMed]
- Kordouni M, Jibrini M, Siddiqui MA. Long-term transvenous temporary pacing with active fixation bipolar lead in the management of severe autonomic dysfunction in Miller-Fisher syndrome: a case report. Int J Cardiol. 2007;117(1):e10–e12. [CrossRef] [PubMed]
- Rastan AJ, Doll N, Walther T, Mohr FW. Pacemaker dependent patients with device infection—a modified approach. Eur J Cardiothorac Surg. 2005;27(6):1116–1118. [CrossRef] [PubMed]
- Braun MU, Rauwolf T, Bock M, et al. Percutaneous Lead Implantation Connected to an External Device in Stimulation-Dependent Patients with systemic Infection—a prospective and controlled study. Pacing Clin Electrophysiol. 2006;29(8):875–879. [CrossRef] [PubMed]
- Chihrin SM, Mohammed U, Yee R, et al. Utility and cost effectiveness of temporary pacing using active fixation leads and an externally placed reusable permanent pacemaker. Am J Cardiol. 2006;98(12):1613–1615. [CrossRef] [PubMed]
- Kornberger A, Schmid E, Kalender G, et al. Bridge to recovery or permanent system implantation: an eight-year single-center experience in transvenous semipermanent pacing. Pacing Clin Electrophysiol. 2013;36(9):1096–1103. [CrossRef] [PubMed]
- Kawata H, Pretorius V, Phan H, et al. Utility and safety of temporary pacing using active fixation leads and externalized re-usable permanent pacemakers after lead extraction. Europace. 2013;15(9):1287–1291. [CrossRef] [PubMed]
- Lever N, Ferguson JD, Bashir Y, Channon KM. Prolonged temporary cardiac pacing using subcutaneous tunneled active-fixation permanent pacing leads. Heart. 2003;89(2):209–210. [CrossRef] [PubMed]
- Lepillier A, Otmani A, Waintraub X, Ollitrault J, Heuzey JL, Lavergne T. Temporary transvenous VDD pacing as a bridge to permanent pacemaker implantation in patients with sepsis and haemodinamically significant atrioventricular block. Europace. 2012;14(7):981–985. [PubMed]
- Pang B, Everest E, McGavigan AD. Utility of atrial temporary pacing as an acute treatment for bradyarrhythmias and tachyarrhythmias in the intensive care setting with preservation of atrioventricular synchrony. Intern Med J. 2012;42(5):581–585. [CrossRef] [PubMed]
- Orsbourn G, Lever N, Harding SA. Use of tunneled active fixation leads allows reliable temporary pacing over prolonged periods. Intern Med J. 2008;38(9):735–738. [CrossRef] [PubMed]
- Cooper JM, Lavi N, Polin GM, Dixit S, Verdino RJ. Temporary external implantable cardioverter defibrillator in the pacemaker-dependent ventricular tachycardia patient. Europace. 2011;13(5):761–763. [CrossRef] [PubMed]
- Jokhi PP, Kalra PR, Radvan J. Emergency transvenous temporary cardiac pacing using an active-fixation permanent pacemaker electrode. Int J Cardiol. 2005;102(3):543–544. [CrossRef] [PubMed]
- Swerdlow CD, Wang PJ, Zipes DP. Pacemakers and implantable cardioverter-defibrillators. In: Mann DL, Zipes DP, Libby P, Bonow RO, Braunwald E, eds. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. Philadelphia, PA: Elsevier; 2015:721–747.
- Weinstein J, Gnoj J, Mazzara JT, Ayres SM, Grace WJ. Temporary transvenous pacing via the percutaneous femoral vein approach. A prospective study of 100 cases. Am Heart J. 1973;85(5):695–705. [CrossRef] [PubMed]
- Winner S, Boon N. Clinical problems with temporary pacemakers prior to permanent pacing. J R Coll Physicians Lond. 1989;23(3):161–163. [PubMed]
- Andrews R, Skehan JD. Temporary pacing: continuing failures in medical management. Br Heart J. 1992;68:91.
- Lang CC, Grubb NR. Hybrid long-term temporary pacing. J Invasive Cardiol. 2005;17(6):338–339. [PubMed]
- Maciag A, Syska P, Oręziak A, et al. Long-term temporary pacing with an active fixation lead. Kardiol Pol. 2015;73(12):1304–1309. [CrossRef] [PubMed]
- Aljabri K, Garlitski A, Weinstock J, Madias C. Management of device infections. Card Electrophysiol Clin. 2018;10(1):153–162. [CrossRef] [PubMed]
- Nandyala R, Parsonnet V. One stage side-to-side replacement of infected pulse generators and leads. Pacing Clin Electrophysiol. 2006;29(4):393–396. [CrossRef] [PubMed]
- Mountantonakis SE, Tschabrunn CM, Deyell MW, Cooper JM. Same-day contralateral implantation of a permanent device after lead extraction for isolated pocket infection. Europace. 2014;16(2):252–257. [CrossRef] [PubMed]
- Amraoui S, Barandon L, Whinnett Z, et al. Single surgical procedure combining epicardial pamcemaker implantation and subsequent extraction of the infected pacing system for pacemaker-dependent patients. J Thorac Cardiovasc Surg. 2013;146(2):302–305. [CrossRef] [PubMed]
- Merrer J, De Jonghe B, Golliot F, et al. Complications of femoral and subclavian venous catheterization in critically ill patients: a randomized controlled trial. JAMA. 2001;286(6):700–707. [CrossRef] [PubMed]
- Parienti JJ, Mongardon N, Megarbane B, et al. Intravascular complications of central venous catheters by insertion site. N Engl J Med. 2015;373:1220–1229. [CrossRef]
- Golden GT, Lovett WL, Harrah JD, Wellons HA Jr, Nolan SP. The treatment of extruded and infected permanent cardiac pulse generator: Application of a technique of closed irrigation. Surgery. 1973;74(4):575–579. [PubMed]
- Graham AS, Ozment C, Tegtmeyer K, Lai S, Braner DA. Central venous catheterization. N Eng J Med. 2007;356(21):e21. [CrossRef] [PubMed]
- Ferguson JD, Lever N, Channon KM, Bashir Y. A simplified approach to temporary DDD pacing using a single lead, balloon-tipped catheter with overlapping biphasic impulse stimulation. Pacing Clin Electrophysiol. 2001;24(6):939–944. [CrossRef] [PubMed]
- Arias MA, Puchol A, Pachon M, Jimenez-Lopez J, Rodriguez-Padial L. Prolonged temporary cardiac pacing using an external permanent pacing system. Rev Esp Cardiol (Eng Ed). 2012;65(6):573–574. [CrossRef] [PubMed]
- Noble S, Roffi M, Burri H. [Use of an explanted pacemaker connected to a regular screw-in lead for temporary pacing.] Rev Esp Cardiol. 2011;64(12):1229–1230. Article in Spanish. [CrossRef] [PubMed]
- Dawood FZ, Boerkircher A, Rubery B, Hire D, Soliman EZ. Risk of early mortality after placement of a temporary-permanent pacemaker. J Electrocardiol. 2016;49(4):530–535. [CrossRef] [PubMed]
- Betts TR. Regional survey of temporary transvenous pacing procedures and complications. Postgrad Med J. 2003;79(934):463–465. [CrossRef] [PubMed]
- Cuisset T, Quilici J. Subclavicular screwed wire transient pacing to increase safety of transcatheter aortic valve implantation with the CoreValve system. J Cardiol Cases. 2011;3(3):e167–e169. [CrossRef] [PubMed]
- Webcast Series Collections
- S-ICD Case Collection
- Fluoro Reduction
- Complimentary Subscription

Editorial Board | Author Guidelines | About Us | Contact Us | Submit Manuscript | Subscribe | Terms of Use | Privacy Policy
505 South Lenola Road, Suite 121, Moorestown, NJ 08057
Website Design Internet Marketing by DigitalSequence
Wandering Atrial Pacemaker ECG Interpretation with Sample Strip
Wandering atrial pacemaker rhythm strip features, authors and reviewers.
- ECG heart rhythm modules: Thomas O'Brien.
- ECG monitor simulation developer: Steve Collmann
- 12 Lead Course: Dr. Michael Mazzini, MD .
- Spanish language ECG: Breena R. Taira, MD, MPH
- Medical review: Dr. Jonathan Keroes, MD
- Medical review: Dr. Pedro Azevedo, MD, Cardiology
- Last Update: 11/8/2021
- Electrocardiography for Healthcare Professionals, 6th Edition Kathryn Booth and Thomas O'Brien ISBN10: 1265013470, ISBN13: 9781265013479 McGraw Hill, 2023
- Rapid Interpretation of EKG's, Sixth Edition Dale Dublin Cover Publishing Company
- EKG Reference Guide EKG.Academy
- 12 Lead EKG for Nurses: Simple Steps to Interpret Rhythms, Arrhythmias, Blocks, Hypertrophy, Infarcts, & Cardiac Drugs Aaron Reed Create Space Independent Publishing
- Heart Sounds and Murmurs: A Practical Guide with Audio CD-ROM 3rd Edition Elsevier-Health Sciences Division Barbara A. Erickson, PhD, RN, CCRN
- The Virtual Cardiac Patient: A Multimedia Guide to Heart Sounds, Murmurs, EKG Jonathan Keroes, David Lieberman Publisher: Lippincott Williams & Wilkin) ISBN-10: 0781784425; ISBN-13: 978-0781784429
- Project Semilla, UCLA Emergency Medicine, EKG Training Breena R. Taira, MD, MPH
- ECG Reference Guide PracticalClinicalSkills.com
This website provides professional medical education. For medical care contact your doctor. 2024 ©MedEdu LLC. All Rights Reserved. Terms & Conditions | About Us | Privacy | Email Us | 1
Advertisement

- Previous Issue
- Previous Article
- Next Article
Methodology
Advisory evidence and recommendations, appendix 1: summary of advisory recommendations ‡‡‡‡‡, appendix 2: methods and analyses, practice advisory for the perioperative management of patients with cardiac implantable electronic devices: pacemakers and implantable cardioverter–defibrillators 2020 : an updated report by the american society of anesthesiologists task force on perioperative management of patients with cardiac implantable electronic devices *.
This article is featured in “This Month in Anesthesiology,” page 1A.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are available in both the HTML and PDF versions of this article. Links to the digital files are provided in the HTML text of this article on the Journal’s Web site ( www.anesthesiology.org ).
Received from the American Society of Anesthesiologists, Schaumburg, Illinois. Submitted for publication March 13, 2019. Accepted for publication April 30, 2019. Published online first on December 17, 2019. Corrected on February 5, 2020.
Supported by the American Society of Anesthesiologists and developed under the direction of the Committee on Standards and Practice Parameters, Jeffrey L. Apfelbaum, M.D. (Chair). Approved by the American Society of Anesthesiologists House of Delegates on October 23, 2019. This advisory has been endorsed by the Society of Cardiovascular Anesthesiologists.
A complete bibliography used to develop this updated Advisory, arranged alphabetically by author, is available as Supplemental Digital Content 1 ( https://links.lww.com/ALN/B979 ).
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Cite Icon Cite
- Get Permissions
- Search Site
Practice Advisory for the Perioperative Management of Patients with Cardiac Implantable Electronic Devices: Pacemakers and Implantable Cardioverter–Defibrillators 2020 : An Updated Report by the American Society of Anesthesiologists Task Force on Perioperative Management of Patients with Cardiac Implantable Electronic Devices . Anesthesiology 2020; 132:225–252 doi: https://doi.org/10.1097/ALN.0000000000002821
Download citation file:
- Ris (Zotero)
- Reference Manager
Practice advisories are systematically developed reports that are intended to assist decision-making in areas of patient care. Advisories provide a synthesis of scientific literature and analysis of expert opinion, clinical feasibility data, open forum commentary, and consensus surveys. Practice advisories developed by the American Society of Anesthesiologists (ASA) are not intended as standards, guidelines, or absolute requirements, and their use cannot guarantee any specific outcome. They may be adopted, modified, or rejected according to clinical needs and constraints, and they are not intended to replace local institutional policies.
Practice advisories summarize the state of the literature and report opinions obtained from expert consultants and ASA members. They are not supported by scientific literature to the same degree as standards or guidelines because of the lack of sufficient numbers of adequately controlled studies. Practice advisories are subject to periodic revision as warranted by the evolution of medical knowledge, technology, and practice.
This document updates the Practice Advisory for the Perioperative Management of Patients with Cardiac Implantable Electronic Devices: Pacemakers and Implantable Cardioverter–Defibrillators: An Updated Report by the American Society of Anesthesiologists Task Force on Perioperative Management of Patients with Cardiac Implantable Electronic Devices, adopted by the ASA in 2010 and published in 2011. 1

Definition of Cardiac Implantable Electronic Devices
For this advisory, a cardiac implantable electronic device refers to any permanently implantable cardiac pacemaker or any implantable cardioverter–defibrillator. The term cardiac implantable electronic device also refers to any cardiac resynchronization therapy device. †
Purposes of the Advisory
The purposes of this advisory update are to: (1) facilitate safe and effective perioperative management of the patient with a cardiac implantable electronic device and (2) reduce the incidence of adverse outcomes. Perioperative management refers to the preoperative, intraoperative, postoperative, or recovery period in any setting where an anesthesia provider will be delivering anesthesia care. Adverse outcomes associated with cardiac implantable electronic device function include, but are not limited to, damage to the device, inability of the device to deliver pacing or shocks, lead-tissue interface damage, changes in pacing behavior, electrical reset to the backup pacing mode, and inappropriate implantable cardioverter–defibrillator therapies. ‡
Adverse clinical outcomes include, but are not limited to, hypotension, tachyarrhythmia and bradyarrhythmia, myocardial tissue damage, and myocardial ischemia and infarction. Other related adverse outcomes may include extended hospital stay, delay and cancellation of surgery, readmission to manage device malfunction, and additional hospital resource utilization and cost.
Focus of the Advisory
This updated advisory focuses on the perioperative management of the patient who has a preexisting cardiac implantable electronic device for the treatment of bradyarrhythmia, tachyarrhythmia, or heart failure. This advisory applies to all cardiac implantable electronic device patients receiving general or regional anesthesia, sedation, or monitored anesthesia care. Both inpatient and outpatient procedures are addressed by this update.
This update does not address the perioperative management of the patient without a cardiac implantable electronic device, such as those (1) with only a temporary cardiac implantable electronic device; (2) with only a noncardiac implantable device ( e.g. , neurologic or spinal cord stimulator); (3) with only an implantable mechanical cardiac assist device ( e.g. , ventricular assist device); or (4) undergoing cardiac implantable electronic device implantation or revision. This update does not address procedures rarely involving anesthesia care ( e.g. , radiation therapy § ) or imaging modalities without known perioperative cardiac implantable electronic device concerns ( e.g. , diagnostic radiography or ultrasonography). In addition, this update does not address patient comfort or management of pain during a procedure.
Application of the Advisory
This updated advisory is intended for use by anesthesiologists and all other individuals who deliver or who are responsible for anesthesia care. This update may also serve as a resource for other physicians and healthcare professionals who manage patients with cardiac implantable electronic devices.
Task Force Members and Consultants
The original advisory was developed by an ASA-appointed task force of 12 members consisting of anesthesiologists and cardiologists in private and academic practices from various geographic areas of the United States and two methodologists from the ASA Committee on Standards and Practice Parameters. In 2017, the ASA Committee on Standards and Practice Parameters requested that the advisory be updated. This update is a revision developed by an ASA-appointed task force of five members, including three anesthesiologists and two methodologists. Conflict-of-interest documentation regarding current or potential financial and other interests pertinent to the practice guideline were disclosed by all task force members and managed.
Process and Evaluation of Evidence
This updated advisory was developed by means of a five-step process. First, consensus was reached on the criteria for evidence. Second, original published articles from peer-reviewed journals relevant to the perioperative management of cardiac implantable electronic devices were evaluated and added to literature reported in the previous update. Third, consultants who had expertise or interest in cardiac implantable electronic devices and who practiced or worked in various settings ( e.g. , private and academic practice) were asked to participate in opinion surveys addressing the appropriateness, completeness, and feasibility of implementation of the draft recommendations and to review and comment on a draft of the Advisory. Fourth, additional opinions were solicited from random samples of active ASA members. Fifth, all available information was used to build consensus to finalize the advisory. A summary of recommendations can be found in appendix 1.
Preparation of this updated advisory followed a rigorous methodologic process. Evidence was obtained from two principal sources: scientific evidence and opinion-based evidence. Detailed descriptions of the ASA process and methodology used in this Advisory may be found in other related publications. 2–5 Appendix 2 contains information on the evidence model, the literature search process, literature findings, and survey results.
Within the text of the advisory, literature classifications are reported for each intervention using the following classifications: category A, level 1: meta-analysis of randomized controlled trials; category A, level 2, multiple randomized controlled trials; category A, level 3: a single randomized controlled trial; category B, level 1: nonrandomized studies with group comparisons; category B, level 2: nonrandomized studies with associative findings; category B, level 3: nonrandomized studies with descriptive findings; and category B, level 4: case series or case reports. Outcomes are designated as either beneficial (B) or harmful (H) for the patient; statistically nonsignificant findings are designated as equivocal (E). Survey findings from task force–appointed expert consultants and a random sample of the ASA membership are fully reported in the text of these guidelines. Survey responses for each recommendation are reported using a five-point scale based on median values from “strongly agree” to “strongly disagree.”
Preoperative Evaluation
A focused preoperative evaluation of the patient with a cardiac implantable electronic device consists of the following topics: (1) determining whether a patient has a cardiac implantable electronic device; (2) determining the cardiac implantable electronic device type, manufacturer, and primary indication for placement; (3) determining whether a patient is pacing-dependent; and (4) determining the cardiac implantable electronic device’s current settings and that it is functioning properly by interrogating the cardiac implantable electronic device or obtaining the most recent interrogation report.
Literature Findings. Although the literature is insufficient to evaluate the clinical benefit of performing a focused preoperative evaluation of patients with cardiac implantable electronic devices, case reports indicate that adverse outcomes ( e.g. , inappropriate shock, cardiac implantable electronic device switch to “end-of-life mode,” acute ventricular lead dysfunction, and corrupted device memory) may occur when a complete preoperative examination is not performed to determine whether the patient has a cardiac implantable electronic device (Category B4-H evidence). 6–9 The literature is insufficient to evaluate whether preoperatively determining the cardiac implantable electronic device type, manufacturer, and primary indication for placement or determining whether a patient is pacing-dependent affects perioperative outcomes. A case series reported inappropriate antitachycardia pacing or shocks, premature battery depletion, and cardiac implantable electronic device damage when the cardiac implantable electronic device’s settings were not adequately assessed preoperatively (Category B4-H evidence ). 10 The literature is insufficient to evaluate whether any particular time interval to determine recency for review of a previous cardiac implantable electronic device interrogation is most beneficial to the patient.
Survey Findings. The expert consultants and ASA members strongly agree with the recommendation that a preoperative evaluation should include determining whether a patient has a cardiac implantable electronic device, determining the cardiac implantable electronic device type ( i.e. , pacemaker, implantable cardioverter–defibrillator, cardiac resynchronization therapy), determining the primary indication for cardiac implantable electronic device placement, and determining whether the patient is pacing-dependent. The consultants strongly agree and ASA members agree that a preoperative evaluation should include determining the cardiac implantable electronic device manufacturer.
The consultants strongly agree and ASA members agree that a preoperative evaluation should include determining the cardiac implantable electronic device’s current settings and confirming that the cardiac implantable electronic device is functioning properly ( i.e. , by interrogating the cardiac implantable electronic device or obtaining the most recent interrogation report). The consultants selected preferred time spans for determining proper implantable cardioverter–defibrillator functioning before a procedure, as follows: immediately = 6% of consultants, at least 3 months before = 48% of consultants, at least 6 months before = 36% of consultants, and at least 12 months before = 6% of consultants. For a pacemaker the following time spans were selected by consultants: immediately = 3% of consultants, at least 3 months before = 39% of consultants, at least 6 months before = 30% of consultants, and at least 12 months before = 27% of consultants. The ASA members selected the following preferred time spans for determining proper functioning of an implantable cardioverter–defibrillator before the procedure: immediately = 10% of members, at least 3 months before = 39% of members, at least 6 months before = 44% of members, and at least 12 months before = 7% of members. For a pacemaker the following time spans were selected by members: immediately = 9% of members, at least 3 months before = 38%, at least 6 months before = 36%, and at least 12 months before = 18% of consultants.
Advisory Recommendations for Preoperative Evaluation
Determine whether a patient has a cardiac implantable electronic device
Conduct a focused history ( e.g. , interview the patient or other source, review medical record, chest x-ray, and electrocardiogram if available)
Perform a focused physical examination ( e.g. , check for scars, palpate for device) ‖
Determine the cardiac implantable electronic device type, manufacturer, and primary indication for placement
Obtain the manufacturer’s identification card from the patient or other source
Review the medical record
Obtain and review the most recent cardiac implantable electronic device interrogation report #
Refer to supplemental resources ( e.g. , manufacturer’s databases, cardiac implantable electronic device clinic records)
Order a chest x-ray if no other data are available **
Determine whether the patient is pacing-dependent ††
From the focused history and medical record, assess for one or more of the following indicators:
Bradycardia that caused syncope or other symptoms resulting in cardiac implantable electronic device implantation
Successful atrioventricular nodal ablation resulting in cardiac implantable electronic device implantation
A cardiac implantable electronic device interrogation showing no evidence of spontaneous ventricular activity when the cardiac implantable electronic device’s pacing function is temporarily programmed to a nontracking mode ( i.e. , ventricular-only pacing and sensing) at the lowest programmable rate
Determine the cardiac implantable electronic device’s current settings, that it is functioning properly ( i.e. , by interrogating the cardiac implantable electronic device or obtaining the most recent interrogation report), and that it is optimally programmed for the planned procedure ‡‡ §§
Reinterrogate the cardiac implantable electronic device if there is any question of proper function
Preoperative Preparation
Preoperative preparation for patient safety and proper maintenance of the cardiac implantable electronic device during a planned procedure includes the following topics: (1) sources of electromagnetic interference; (2) preoperative reprogramming of the cardiac implantable electronic device’s pacing function to an asynchronous pacing mode or disabling any special algorithms, including rate adaptive pacing functions; (3) suspending the antitachyarrhythmia functions for an implantable cardioverter–defibrillator; and (4) availability of temporary pacing and defibrillation equipment.
Literature Findings. The literature was evaluated for the following potential sources of electromagnetic interference: monopolar electrosurgery, bipolar electrosurgery, radiofrequency ablation, lithotripsy, external cardioversion or defibrillation, magnetic resonance imaging, radiation therapy, radiofrequency scanners, cardiac monitors, and electroconvulsive therapy.
Observational studies report that electromagnetic interference may occur during monopolar electrosurgery, 11–15 radiofrequency ablation, 16–21 magnetic resonance imaging, 22–35 and radiation therapy 36–42 (Category B3-H evidence). Case reports also indicate the occurrence of electromagnetic interference during monopolar electrosurgery, 43–50 bipolar electrosurgery, 51 radiofrequency ablation, 52–54 magnetic resonance imaging, 6–9 , 55 , 56 and radiation therapy 57–59 (Category B4-H evidence ).
Case reports indicate that inappropriately high pacing rates may occur due to electromagnetic interference from cardiac monitoring equipment in cardiac implantable electronic devices with active minute ventilation sensors (Category B4-H evidence ). 60–62 An observational study of implantable cardioverter–defibrillators in the pectoral position reports a significantly higher occurrence of electromagnetic interference when electrosurgery above the umbilicus is performed compared with electrosurgery below the umbilicus (Category B1-H evidence ). 15 The literature is insufficient to evaluate the benefit of the availability of temporary pacing and defibrillation equipment during a procedure.
Survey Findings. The consultants and ASA members strongly agree that a preoperative evaluation should include determining whether electromagnetic interference from monopolar electrosurgery or other sources is likely to occur and strongly agree with the recommendation to alter the pacing function of a cardiac implantable electronic device to an asynchronous pacing mode in the pacing-dependent patient if monopolar electrosurgery (“bovie”) use is planned superior to the umbilicus. The consultants disagree and ASA members are equivocal with the recommendation to alter the pacing function of a cardiac implantable electronic device to an asynchronous pacing mode in the pacing-dependent patient if monopolar electrosurgery (bovie) use is planned inferior to the umbilicus. The consultants and ASA members strongly agree with the recommendation to suspend an implantable cardioverter–defibrillator’s antitachycardia function, when present, if monopolar electrosurgery (bovie) use is planned superior to the umbilicus. The consultants agree and ASA members are equivocal with the recommendation to suspend an implantable cardioverter–defibrillator’s antitachycardia function, when present, if monopolar electrosurgery (bovie) use is planned inferior to the umbilicus. The consultants and ASA members strongly agree with the recommendation to ensure that the patient is in a monitored environment before suspending the antitachycardia function of an implantable cardioverter–defibrillator. The consultants are equivocal and ASA members agree with the recommendation to avoid the routine use of a magnet over an implantable cardioverter–defibrillator. The consultants and ASA members strongly agree that if needed, a specialist should be consulted to alter the pacing function of a cardiac implantable electronic device or to suspend the antitachycardia function of an implantable cardioverter–defibrillator. The consultants and ASA members strongly agree that the proceduralist should be advised to use bipolar electrosurgery or an ultrasonic scalpel when feasible. The consultants and ASA members strongly agree with the recommendation that temporary pacing and defibrillation equipment should be immediately available before, during, and after all procedures with electromagnetic interference potential. Finally, the consultants and ASA members agree with the recommendation that a cardiac implantable electronic device’s active sensor for rate-responsive pacing should be suspended to prevent undesirable tachycardia.
Advisory Recommendations for Preoperative Preparation
Determine whether intraoperative electromagnetic interference electromagnetic interference is likely to occur
If electromagnetic interference is likely to occur ( e.g. , monopolar electrosurgery [bovie] use, or radiofrequency ablation is planned superior to the umbilicus), alter the pacing function of a cardiac implantable electronic device to an asynchronous pacing mode in the pacing-dependent patient ‖‖ ## and suspend an implantable cardioverter–defibrillator’s antitachycardia function, if present ***
Before suspending the antitachycardia function, ensure that the patient is in a monitored environment
Avoid the indiscriminate use of a magnet over an implantable cardioverter–defibrillator
If needed, consult a specialist to alter the pacing function of a cardiac implantable electronic device or to suspend the antitachycardia function of an implantable cardioverter–defibrillator
Ensure that temporary pacing and defibrillation equipment are immediately available before, during, and after all procedures with electromagnetic interference potential
Suspend a cardiac implantable electronic device’s active sensor for rate-responsive pacing to prevent undesirable tachycardia †††
Intraoperative Monitoring
Intraoperative monitoring topics include (1) continuous electrocardiography monitoring; (2) continuous oxygen saturation measured by pulse oximetry (Sp o 2 ) monitoring; and (3) peripheral pulse monitoring ( e.g. , pulse palpitation, pulse oximeter plethysmogram, or arterial line).
Literature Findings. Case reports indicate that continuous electrocardiography monitoring may detect electromagnetic interference-related pacemaker function abnormalities 49 , 56 , 63 and cardiac abnormalities 64 , 65 during a procedure (Category B4-B evidence). For cardiac implantable electronic device patients, the literature is insufficient to evaluate the clinical impact of continuous Sp o 2 or perioperative peripheral pulse monitoring.
Survey Findings. The consultants and ASA members strongly agree with the recommendations to (1) continuously monitor and display a patient’s electrocardiogram as required by ASA standards from the beginning of anesthesia until the patient is transferred out of the anesthetizing location, with additional electrocardiography monitoring in the postoperative period as indicated by the patient’s medical condition; (2) perform continuous peripheral pulse monitoring for all cardiac implantable electronic device patients receiving anesthesia care; and (3) discontinue the procedure until the source of interference can be eliminated or managed if unanticipated cardiac implantable electronic device interactions occur.
Advisory Recommendations for Intraoperative Monitoring
Continuously monitor and display a patient’s electrocardiogram and Sp o 2 as required by ASA standards 66 , 67 from the beginning of anesthesia until the patient is transferred out of the anesthetizing location ‡‡‡
Perform continuous peripheral pulse monitoring for all cardiac implantable electronic device patients receiving anesthesia care §§§
If unanticipated cardiac implantable electronic device interactions occur, temporarily suspend the procedure until the source of interference can be identified and eliminated or managed
Managing Potential Sources of Electromagnetic Interference
Procedures using electrosurgery, radiofrequency ablation, radiofrequency identification devices, lithotripsy, magnetic resonance imaging, radiation therapy, nerve conduction studies, cardioversion, or electroconvulsive therapy may damage cardiac implantable electronic devices or interfere with cardiac implantable electronic device function, potentially resulting in severe adverse outcomes. Sources of electromagnetic interference are often unique to specific procedures, and the management of each of these potential electromagnetic interference sources is reported separately below.
Electrosurgery
Management of potential sources of electromagnetic interference associated with electrosurgery includes the following topics: (1) positioning the electrosurgical unit’s dispersive electrode so that the current pathway does not pass through or near the cardiac implantable electronic device generator and leads; (2) avoiding proximity of the electrosurgical unit’s electrical current to the generator or leads; (3) using intermittent and irregular bursts of monopolar electrosurgery at the lowest feasible energy levels; (4) using bipolar electrosurgery; and (5) using ultrasonic (harmonic) scalpel.
Literature Findings. The literature is insufficient to evaluate whether positioning the current pathway away from the cardiac implantable electronic device generator and leads reduces the occurrence of electromagnetic interference. A case report indicates that electromagnetic interference occurred when the electrosurgical unit’s electrical current was placed in proximity to the generator or leads (Category B4-H evidence ). 68 An observational study reports that electromagnetic interference may occur in spite of positioning the dispersive electrode to divert the return path away from the generator and leads (Category B3-H evidence ). 15 Case reports also indicate that electromagnetic interference may still occur when proximity is avoided (Category B4-H evidence ). 46 , 66 No controlled studies were found that examine the benefit of using short intermittent bursts of electrosurgery at the lowest feasible energy levels. One case report describes pacemaker failure when short bursts of current were used with a bipolar electrosurgery system (Category B4-H evidence). 51
Case reports indicate that cardiac arrhythmias and asystole occurred when monopolar electrosurgery was initiated, and after changing to bipolar electrosurgery, the procedures proceeded uneventfully (Category B4-B evidence ). 46 , 64 , 65 A case report indicated that dysrhythmias followed by asystole occurred when monopolar electrosurgery was initiated, and after changing to a harmonic scalpel, the procedure was completed successfully (Category B4-B evidence ). 44
Survey Findings. The consultants and ASA members strongly agree with the recommendations to (1) minimize the risk of electromagnetic interference by positioning the electrosurgical instrument and dispersive electrode (bovie pad) so the current pathway does not pass through or near the cardiac implantable electronic device generator or leads; (2) avoid proximity of the electrosurgery electrical field to the generator and leads, including the avoidance of waving the activated electrode over the generator; and (3) use short, intermittent, and irregular bursts of electrosurgery at the lowest feasible energy levels. The consultants agree and ASA members strongly agree with the recommendations to use bipolar electrosurgery or an ultrasonic (harmonic) scalpel, if possible.
Radiofrequency Ablation
Management of potential sources of electromagnetic interference associated with radiofrequency ablation primarily involves keeping the radiofrequency current path (electrode tip to current return pad) as far away from the generator and leads as possible.
Literature Findings. The literature is insufficient to examine the benefit of avoiding direct contact between the ablation catheter and the generator and leads or of keeping the radiofrequency current path (electrode tip to current return pad) as far away from the generator and leads as possible.
Survey Findings. The consultants and ASA members strongly agree with the recommendations to avoid direct contact between the ablation catheter and the generator and leads and to keep the radiofrequency’s current path (electrode tip to current return pad) as far away from the generator and leads as possible.
Lithotripsy
Management of potential sources of electromagnetic interference associated with lithotripsy consists of avoiding focus of the lithotripsy beam near the generator.
Literature Findings. The literature insufficient to evaluate the benefits of focusing the lithotripsy beam away from the generator.
Survey Findings. The consultants and ASA members strongly agree with the recommendation to avoid focusing the lithotripsy beam near the generator.
Magnetic Resonance Imaging
Management of potential sources of electromagnetic interference associated with magnetic resonance imaging include the topics of (1) moving the patient outside of the immediate magnetic resonance imaging area when an external defibrillator/monitor, cardiac implantable electronic device programmer, or any other magnetic resonance imaging-unsafe equipment is used; (2) interrogating the cardiac implantable electronic device before the magnetic resonance imaging scan; (3) suspending the antitachycardia function of an implantable cardioverter–defibrillator before the magnetic resonance imaging scan; (4) altering the pacing function of the cardiac implantable electronic device to an asynchronous pacing mode in the pacing-dependent patient before the magnetic resonance imaging scan; (5) ensuring that an individual capable of programming the cardiac implantable electronic device remains in attendance for the duration of the magnetic resonance imaging scan; and (6) reinterrogating the cardiac implantable electronic device and restoring its permanent settings after the magnetic resonance imaging is completed. ‖‖‖
Literature Findings. Observational studies evaluating the effects of suspending the antitachycardia function of an implantable cardioverter–defibrillator report that electromagnetic interference may still occur (Category B3-E evidence ). 25 , 30 , 32 , 33 Observational studies of magnetic resonance imaging-conditional cardiac implantable electronic devices report that electromagnetic interference does not occur when a cardiac implantable electronic device is programed to “magnetic resonance imaging mode” and the antitachycardia function is suspended (Category B3-E evidence ). 22–24
The literature is insufficient to examine the necessity of: (1) moving the patient outside of the magnetic resonance imaging area when an external defibrillator/monitor, cardiac implantable electronic device programming system, or any other magnetic resonance imaging-unsafe equipment is used; (2) interrogating a cardiac implantable electronic device before magnetic resonance imaging is performed; (3) having an individual capable of programming the cardiac implantable electronic device remain in attendance for the duration of magnetic resonance imaging; and (4) reinterrogating the cardiac implantable electronic device and restoring its permanent settings after magnetic resonance imaging is completed.
Survey Findings. The consultants and ASA members strongly agree with the recommendations to move the patient outside of the immediate magnetic resonance imaging area when the use of an external defibrillator/monitor, cardiac implantable electronic device programmer, or any other magnetic resonance imaging-unsafe equipment is required and to monitor the patient’s electrocardiogram and/or Sp o 2 continuously throughout the magnetic resonance imaging. The consultants agree and ASA members are equivocal regarding the recommendation to have an individual capable of programming the cardiac implantable electronic device remain in attendance for the duration of the magnetic resonance imaging.
For magnetic resonance imaging-conditional cardiac implantable electronic devices, the consultants strongly agree and ASA members agree with the recommendations to interrogate a cardiac implantable electronic device, program the cardiac implantable electronic device to magnetic resonance imaging mode, suspend the antitachycardia function of an implantable cardioverter–defibrillator, and alter the pacing function of the cardiac implantable electronic device to an asynchronous pacing mode in the pacing-dependent patient before the magnetic resonance imaging. The consultants and ASA members strongly agree with the recommendation to reinterrogate the cardiac implantable electronic device and restore its permanent settings after the magnetic resonance imaging scan.
For magnetic resonance imaging nonconditional cardiac implantable electronic devices, the consultants strongly agree and ASA members agree with the recommendations to interrogate a cardiac implantable electronic device before the magnetic resonance imaging scan, alter the pacing function of the cardiac implantable electronic device to an asynchronous pacing mode in the pacing-dependent patient, and suspend the antitachycardia function of an implantable cardioverter–defibrillator if present. The consultants and ASA members strongly agree with the recommendation to reinterrogate the cardiac implantable electronic device and restore its permanent settings after the magnetic resonance imaging scan.
Radiofrequency Identification Devices
Radiofrequency identification devices are scanners used to detect retained surgical items. Management of potential sources of electromagnetic interference associated with radiofrequency identification devices addresses the topic of avoiding the use of these devices in close proximity to a cardiac implantable electronic device.
Literature Findings. The literature is insufficient to evaluate either the impact of radiofrequency identification devices as a source of electromagnetic interference or to evaluate whether electromagnetic interference depends on the distance between the radiofrequency source and cardiac implantable electronic device in the perioperative setting.
Survey Findings. For radiofrequency identification devices, the consultants strongly agree and ASA members agree with the recommendations to avoid using radiofrequency identification devices in close proximity to the cardiac implantable electronic device whenever possible.
Electroconvulsive Therapy
Management of potential sources of electromagnetic interference associated with electroconvulsive therapy includes the topics of altering the pacing function of a cardiac implantable electronic device to an asynchronous pacing mode in the pacing-dependent patient, suspending an implantable cardioverter–defibrillator’s antitachycardia functions, and monitoring and treating ventricular arrhythmias that may occur secondary to the hemodynamic effects of electroconvulsive therapy.
Literature Findings. The literature is insufficient to evaluate the effects of specific management activities related to electroconvulsive therapy.
Survey Findings. The consultants and ASA members agree with the recommendations to alter the pacing function of a cardiac implantable electronic device to an asynchronous pacing mode in the pacing-dependent patient and to suspend an implantable cardioverter–defibrillator’s antitachycardia functions, if present. The consultants and ASA members strongly agree with the recommendation to monitor for and treat ventricular arrhythmias that may occur secondary to the hemodynamic effects of electroconvulsive therapy.
Advisory Recommendations for Managing Potential Sources of Electromagnetic Interference
If monopolar electrosurgery is planned superior to the umbilicus, ensure that the pacing function of a cardiac implantable electronic device is altered to an asynchronous pacing mode in the pacing-dependent patient and suspend an implantable cardioverter–defibrillator’s antitachycardia function, if present
Before suspending the antitachycardia function of an implantable cardiovertor defibrillator, ensure that the patient is in a monitored environment
Minimize the risk of electromagnetic interference from monopolar electrosurgery
Position the electrosurgical instrument and dispersive electrode (bovie pad) so the current pathway does not pass through or near the cardiac implantable electronic device generator or leads ### ****
Avoid waving the activated electrode over the generator ††††
Use short, intermittent, and irregular bursts of electrosurgery at the lowest feasible energy levels
Use bipolar electrosurgery or an ultrasonic (harmonic) scalpel, if possible
If radiofrequency ablation is planned superior to the umbilicus, ensure that the pacing function of a cardiac implantable electronic device is altered to an asynchronous pacing mode in the pacing-dependent patient and suspend an implantable cardioverter–defibrillator’s antitachycardia function, if present
Avoid direct contact between the ablation catheter and the generator and leads
Keep the radiofrequency’s current path (electrode tip to current return pad) as far away from the generator and leads as possible
Do not focus the lithotripsy beam near the generator
Magnetic Resonance Imaging ‡‡‡‡
Ensure that a standardized workflow and/or institutional protocol is in place and followed
Move the patient outside of the immediate magnetic resonance imaging area when the use of an external defibrillator/monitor, cardiac implantable electronic device programmer, or any other magnetic resonance imaging-unsafe equipment is required
Before the magnetic resonance imaging scan, perform the following:
Interrogate the cardiac implantable electronic device
Suspend the antitachycardia function of an implantable cardioverter–defibrillator, if present
For magnetic resonance imaging-conditional cardiac implantable electronic devices, adhere to all product labeling including activating magnetic resonance imaging mode to suspend the antitachycardia function of a magnetic resonance imaging-conditional implantable cardioverter–defibrillator §§§§
In the pacing-dependent patient, alter the pacing function of the cardiac implantable electronic device to an asynchronous pacing mode
Ensure that an individual capable of performing advanced cardiac life support remains in attendance for the duration of the magnetic resonance imaging scan.
Ensure that an individual capable of programming the cardiac implantable electronic device is readily available for consultation or remains in attendance for the duration of the magnetic resonance imaging scan whenever dictated by institutional policy
After the magnetic resonance imaging scan is completed, reinterrogate the cardiac implantable electronic device and restore its permanent settings
Avoid using radiofrequency identification devices in close proximity to the cardiac implantable electronic device whenever possible
Monitor for signs of electromagnetic interference and be prepared to stop using the radiofrequency identification device if interference occurs
Alter the pacing function of a cardiac implantable electronic device to an asynchronous pacing mode in the pacing-dependent patient
Suspend an implantable cardioverter–defibrillator’s antitachycardia function, if present
Monitor for and be prepared to manage postconvulsive sinus tachycardia
Monitor for and treat ventricular arrhythmias that may occur secondary to the hemodynamic effects of electroconvulsive therapy
Emergency External Cardioversion or Defibrillation
During the perioperative period, the cardiac implantable electronic device patient might require emergency external defibrillation or cardioversion. In this case, a concern is to minimize the current flowing through the pulse generator and leads.
Literature Findings. The literature is insufficient to evaluate the effects of specific management activities related to emergency defibrillation or cardioversion.
Survey Findings. The consultants and ASA members agree with the recommendation that before emergently defibrillating or cardioverting a patient with an implantable cardioverter–defibrillator and magnet-disabled therapies, all sources of electromagnetic interference should be terminated, and the magnet should be removed to reenable the implantable cardioverter–defibrillator’s antitachycardia therapies; then the patient should be observed for the delivery of appropriate antitachycardia therapy from the implantable cardioverter–defibrillator. The consultants agree and ASA members strongly agree with the recommendation to determine whether the antitachycardia therapy of an implantable cardioverter–defibrillator should be reenabled when it has been disabled by programming. The consultants and ASA members strongly agree that if the above activities fail to restore implantable cardioverter–defibrillator antitachycardia function, emergency external defibrillation or cardioversion should be performed when needed using advanced cardiac life support guidelines for delivered energy level and pad placement. The consultants and ASA members strongly agree with the recommendation to minimize the current flowing through the generator and leads by positioning the defibrillation or cardioversion pads so they are not directly over the cardiac implantable electronic device. The consultants strongly agree and ASA members agree with the recommendation to use anterior–posterior rather than anterior–lateral pad positioning whenever possible. The consultants and ASA members strongly agree with the recommendations to use a clinically appropriate energy output regardless of the presence of the cardiac implantable electronic device and to interrogate the cardiac implantable electronic device immediately after external cardioversion or defibrillation is performed.
Advisory Recommendations for Emergency Cardioversion or Defibrillation
Before attempting to emergently externally cardiovert or defibrillate a patient with an implantable cardioverter–defibrillator and magnet-disabled therapies, terminate all sources of electromagnetic interference and remove the magnet to reenable the implantable cardioverter–defibrillator’s antitachycardia therapies
Observe the patient for appropriate antitachycardia therapy from the implantable cardioverter–defibrillator
Determine the need for reenabling an implantable cardioverter–defibrillator’s antitachycardia therapy if it was disabled by programming
If the above activities fail to restore the implantable cardioverter’s antitachycardia therapy, or if the antitachycardia therapy cannot be restored expeditiously, proceed with emergency external cardioversion or defibrillation when needed.
Follow advanced cardiac life support guidelines for delivered energy level and pad placement
Position the cardioversion and defibrillation pads so they are not directly over the cardiac implantable electronic device generator to minimize the current flowing through the generator and leads
Use a clinically appropriate energy output regardless of the presence of a cardiac implantable electronic device
Interrogate the cardiac implantable electronic device immediately after external cardioversion or defibrillation is performed
Postoperative Management
Postoperative management of cardiac implantable electronic device patients primarily consists of interrogating and restoring cardiac implantable electronic device function.
Literature Findings. An observational study reports that postoperative interrogation revealed cardiac implantable electronic device malfunctions that occurred during a procedure (Category B3-B evidence). 41 Case reports also indicate that postoperative interrogation may have revealed intraoperative changes to cardiac implantable electronic device settings; subsequently the devices were reprogrammed to their original settings, except in one case where the device was damaged to the point it had to be replaced (Category B4-B evidence ). 10 , 52 The literature is insufficient to evaluate the benefits of: (1) continuing to monitor and display a patient’s electrocardiogram; (2) monitoring cardiac rate and rhythm throughout the immediate postoperative period; (3) ensuring that back-up pacing and cardioversion–defibrillation equipment are immediately available; and (4) restoring the cardiac implantable electronic device to its permanent setting before the patient is discharged from a monitored environment when the cardiac implantable electronic device has been reprogrammed pre- or intraoperatively.
Survey Findings. The consultants and ASA members strongly agree with the following recommendations: (1) continuously monitor cardiac rate and rhythm throughout the immediate postoperative period; (2) for a cardiac implantable electronic device that was reprogrammed pre- or intraoperatively, ensure that back-up pacing and cardioversion–defibrillation equipment is immediately available until the permanent settings are restored; (3) for a cardiac implantable electronic device that was reprogrammed pre- or intraoperatively, restore the cardiac implantable electronic device to its permanent settings before the patient is discharged from a monitored environment; (4) if interrogation determines that the cardiac implantable electronic device settings are inappropriate, then reprogram the cardiac implantable electronic device to newly appropriate settings; (5) perform a postoperative cardiac implantable electronic device interrogation if emergency surgery occurred without appropriate preoperative cardiac implantable electronic device evaluation; (6) perform a postoperative cardiac implantable electronic device interrogation if there is suspicion that antitachycardia therapy might have been disabled rather than temporarily suspended with magnet placement; (7) perform a postoperative cardiac implantable electronic device interrogation if significant electromagnetic interference occurred in close proximity to the cardiac implantable electronic device; and (8) perform a postoperative cardiac implantable electronic device interrogation if the delivery of antitachycardia therapy was observed or if there is concern for cardiac implantable electronic device malfunction. The consultants strongly agree and ASA members agree that if the cardiac implantable electronic device is not interrogated during the immediate postoperative period, interrogate it within 30 days after the procedure.
Advisory Recommendations for Postoperative Management
Continue to monitor and display a patient’s cardiac rate and rhythm throughout the immediate postoperative period as required by ASA standards and as indicated by the patient’s medical condition
For a cardiac implantable electronic device that was reprogrammed pre- or intraoperatively:
Ensure that back-up pacing and cardioversion–defibrillation equipment are immediately available until the cardiac implantable electronic device’s permanent settings are restored ‖‖‖‖
Ensure that the patient’s cardiac rate and rhythm are continuously monitored and displayed until the cardiac implantable electronic device’s permanent settings are restored ####
Ensure that the patient remains in a monitored environment until the cardiac implantable electronic device’s permanent settings are restored ( e.g. , until the antitachycardia function of an implantable cardioverter–defibrillator is reenabled)
Perform a postoperative cardiac implantable electronic device interrogation whenever:
Emergency surgery occurs without appropriate preoperative cardiac implantable electronic device evaluation
There is suspicion that antitachycardia therapy might have been disabled rather than temporarily suspended with magnet placement *****
The delivery of antitachycardia therapy was observed or suspected
There is concern for cardiac implantable electronic device malfunction ( i.e. , significant electromagnetic interference occurred in close proximity to the cardiac implantable electronic device, an invasive procedure was performed in close proximity to a cardiac implantable electronic device generator or lead, or large fluid shifts occurred)
If interrogation determines that the cardiac implantable electronic device settings are inappropriate, reprogram to newly appropriate settings †††††
Determine whether a patient has a cardiac implantable electronic device (cardiac implantable electronic device)
Perform a focused physical examination ( e.g. , check for scars, palpate for device) §§§§§
Obtain and review the most recent cardiac implantable electronic device interrogation report ‖‖‖‖‖
Order a chest x-ray if no other data are available #####
Determine whether the patient is pacing-dependent ******
A cardiac implantable electronic device interrogation showing no evidence of spontaneous ventricular activity when the cardiac implantable electronic device’s pacing function is temporarily programed to a nontracking mode ( i.e. , ventricular-only pacing and sensing) at the lowest programmable rate
Determine the cardiac implantable electronic device’s current settings, that it is functioning properly ( i.e. , by interrogating the cardiac implantable electronic device or obtaining the most recent interrogation report), and that it is optimally programed for the planned procedure ‡‡‡‡‡‡ §§§§§§
Determine whether intraoperative electromagnetic interference is likely to occur.
If electromagnetic interference is likely to occur ( e.g. , monopolar electrosurgery [“bovie”] use, or radiofrequency ablation is planned superior to the umbilicus), alter the pacing function of a cardiac implantable electronic device to an asynchronous pacing mode in the pacing-dependent patient ‖‖‖‖‖‖ ###### and suspend an implantable cardioverter–defibrillator’s antitachycardia function, if present *******
Suspend a cardiac implantable electronic device’s active sensor for rate-responsive pacing to prevent undesirable tachycardia †††††††
Continuously monitor and display a patient’s electrocardiogram and Sp o 2 as required by American Society of Anesthesiologists (ASA) standards 66 , 67 from the beginning of anesthesia until the patient is transferred out of the anesthetizing location ‡‡‡‡‡‡‡
Perform continuous peripheral pulse monitoring for all cardiac implantable electronic device patients receiving anesthesia care §§§§§§§
Position the electrosurgical instrument and dispersive electrode (“bovie pad”) so the current pathway does not pass through or near the cardiac implantable electronic device generator or leads ‖‖‖‖‖‖‖ #######
Avoid waving the activated electrode over the generator ********
Magnetic Resonance Imaging ††††††††
For magnetic resonance imaging-conditional cardiac implantable electronic devices, adhere to all product labeling including activating “magnetic resonance imaging mode” to suspend the antitachycardia function of a magnetic resonance imaging-conditional implantable cardioverter–defibrillator ‡‡‡‡‡‡‡‡
Ensure that an individual capable of performing advanced cardiac life support remains in attendance for the duration of the magnetic resonance imaging scan
Avoid using radiofrequency identification devices in close proximity to the cardiac implantable electronic device, whenever possible
Emergency Cardioversion or Defibrillation
Ensure that back-up pacing and cardioversion–defibrillation equipment are immediately available until the cardiac implantable electronic device’s permanent settings are restored §§§§§§§§
Ensure the patient’s cardiac rate and rhythm are continuously monitored and displayed until the cardiac implantable electronic device’s permanent settings are restored ‖‖‖‖‖‖‖‖
Ensure the patient remains in a monitored environment until the cardiac implantable electronic device’s permanent settings are restored ( e.g. , until the antitachycardia function of an implantable cardioverter–defibrillator is reenabled)
Emergency surgery occurred without appropriate preoperative cardiac implantable electronic device evaluation
There is suspicion that antitachycardia therapy might have been disabled rather than temporarily suspended with magnet placement ########
If interrogation determines that the cardiac implantable electronic device settings are inappropriate, reprogram to newly appropriate settings *********
For this updated practice advisory, a systematic search and review of peer-reviewed published literature was conducted, with scientific findings summarized and reported below and in the document. Assessment of conceptual issues and the practicality and feasibility of the advisory recommendations were also evaluated, with opinion data collected from surveys and other sources. Both the systematic literature review and the opinion data are based on evidence linkages or statements regarding potential relationships between perioperative interventions and electromagnetic interference (electromagnetic interference) outcomes associated with cardiac implantable electronic devices (cardiac implantable electronic devices). The evidence linkage interventions are listed below. The evidence model below guided the search, providing inclusion and exclusion information regarding patients, procedures, practice settings, providers, clinical interventions, and outcomes. After review of all evidentiary information, the task force placed each recommendation into one of three categories: (1) provide the intervention or treatment; (2) the intervention or treatment may be provided to the patient based on circumstances of the case and the practitioner’s clinical judgment; or (3) do not provide the intervention or treatment. The American Society of Anesthesiologists (ASA) Committee on Standards and Practice Parameters reviews all practice parameters at the ASA annual meeting and determines update and revision timelines. The policy of the ASA Committee on Standards and Practice Parameters is to update practice guidelines every 5 yr.
Evidence Model
Inclusion criteria:
Patients with permanently implanted cardiac implantable electronic device for treatment of a bradyarrhythmia, tachyarrhythmia, or heart failure
Implantable cardioverter–defibrillators
Cardiac resynchronization devices
Exclusion criteria:
Patients undergoing cardiac implantable electronic device implantation or revision
Patients without a permanently implantable pacemaker or implantable cardioverter–defibrillator
Patients with a temporary cardiac implantable electronic device
Inpatient procedures
Outpatient procedures
Procedures without known perioperative cardiac implantable electronic device-related concerns
Plain radiography
Fluoroscopy
Practice Settings
Any perioperative setting in which an anesthesia provider will be delivering anesthesia care
Preoperative settings
Intraoperative settings
Postoperative settings
Recovery settings
Nonperioperative settings
Anesthesia care providers
Anesthesiologists
All other individuals who deliver or are responsible for anesthesia care
Individuals who do not deliver or are responsible for anesthesia care
Interventions
Preoperative patient evaluation
Establish whether a patient has a cardiac implantable device
Conduct a focused history
Obtain manufacturer’s identification card from patient or other source
Order chest x-ray if no other data are available
Refer to supplemental resources ( e.g. , manufacturer’s databases)
Determine cardiac implantable electronic device dependency
Determine cardiac implantable electronic device function
Determine whether a cardiac implantable electronic device will capture when it paces
Contact the manufacturer
Preoperative preparation
Determine whether electromagnetic interference occurs during procedure
Radiofrequency ablation
External cardioversion or defibrillation
Magnetic resonance imaging
Electroconvulsive therapy
Determine whether reprogramming a cardiac implantable electronic device to an asynchronous pacing mode is needed
Program antitachyarrhythmia therapy off
Temporary pacing and cardioversion and defibrillation equipment immediately available
bipolar electrosurgery or ultrasonic scalpel
Intraoperative management
Monitor operation of the cardiovascular device
Electrocardiography monitoring (per ASA standard)
Monitor pulse wave form ( e.g. , pulse oximeter plethysmogram, intraarterial pressure)
Management of potential cardiac implantable electronic device dysfunction due to electromagnetic interference
Position the dispersive electrode so that the current pathway does not pass through or near the cardiac electronic device generator and leads
Avoid direct contact with the generator or leads
Use short, intermittent, and irregular bursts at the lowest feasible energy levels
Use bipolar electrosurgery system or ultrasonic scalpel
Use an ultrasonic (harmonic) scalpel (an ultrasonic scalpel can be safely used without affecting a pacemaker or implantable cardioverter–defibrillator)
Keep the current path as far away from the generator and leads as possible
Avoid proximity of the ablation catheter to the leads (intercardiac ablative procedures)
Avoid focusing the lithotripsy beam near the pulse generator
Move the patient outside of the immediate magnetic resonance imaging scan area when the use of an external monitor or cardioverter defibrillator, cardiac implantable electronic device programmer, or any other magnetic resonance imaging-unsafe equipment is required
Monitor the patient’s electrocardiogram and/or Sp o 2 continuously throughout the magnetic resonance imaging scan
An individual capable of programming the cardiac implantable electronic device remaining in attendance for the duration of the magnetic resonance imaging scan
Magnetic resonance imaging-conditional cardiac implantable electronic devices
Before the magnetic resonance imaging, interrogate the cardiac implantable electronic device and program to “magnetic resonance imaging mode” to suspend the antitachycardia function or an implantable cardioverter–defibrillator
Alter the pacing function of the cardiac implantable electronic device to an asynchronous pacing mode in the pacing-dependent patient
Magnetic resonance imaging nonconditional cardiac implantable electronic devices
Interrogate the cardiac implantable electronic device before and after the magnetic resonance imaging scan
Reprogram the cardiac implantable electronic device to an asynchronous pacing mode in the pacing-dependent patient
Radiofrequency identification devices
Avoid using this equipment in close proximity to the cardiac implantable electronic device whenever possible
Monitor for signs of interference with the cardiac implantable electronic device and be prepared to stop using the radiofrequency identification device if interference occurs
Suspend an implantable cardioverter–defibrillator’s antitachycardia therapy, if present
Emergency cardioversion or defibrillation
Patients with an implantable cardioverter–defibrillator and magnet-disabled therapies:
Remove the magnet to reenable antitachycardia therapy
Terminate all sources of electromagnetic interference after magnet is removed
Observe the patient for appropriate cardiac implantable electronic device antitachycardia therapy
Patients with an implantable cardioverter–defibrillator and antitachycardia therapy that have been disabled by programming
Reenable antitachycardia therapy via programming
Minimize the current flowing through the generator and leads
Position cardioversion and defibrillation pads as far as possible from the pulse generator
Use anterior–posterior position
Use a clinically appropriate energy output
Postoperative management
Confirm or restore cardiac implantable electronic device function
Interrogate the implantable cardiac electronic device
Reprogram the implantable cardiac electronic device to appropriate settings
Restore therapy antiarrhythmic therapies
Patients with disabled implantable cardioverter–defibrillator antitachycardia functions
Continuously monitor cardiac function
Keep external cardioversion and defibrillation equipment immediately available until antitachycardia function has been restored
Expected benefits:
Successful procedure
Reduced frequency/severity of adverse outcomes:
Adverse outcomes associated with a cardiac implantable electronic device
Inability to deliver pacing or shocks
Lead-tissue interface damage
Changes in pacing behavior
Electrical reset to the backup pacing mode
Inappropriate implantable cardioverter–defibrillator antitachycardia therapy
Adverse clinical outcomes
Hypotension
Tachyarrhythmia
Bradyarrhythmia
Myocardial tissue damage
Evidence Collection
Literature inclusion criteria:
Randomized controlled trials
Prospective nonrandomized comparative studies ( e.g. , quasi-experimental, cohort)
Retrospective comparative studies ( e.g. , case-control)
Observational studies ( e.g. , correlational or descriptive statistics)
Case reports, case series
Literature exclusion criteria (except to obtain new citations):
Literature reviews
Meta-analyses conducted by others
Unpublished studies
Studies in non–peer-review journals
Newspaper articles
Survey evidence:
Expert consultant survey
ASA membership survey
Other participating organization surveys
Reliability survey
Feasibility survey
State of the Literature
For the systematic review, potentially relevant clinical studies were identified via electronic and manual searches. Healthcare database searches included PubMed, EMBASE, Web of Science, Google Books, and the Cochrane Central Register of Controlled Trials. The searches covered a 9.5-yr period from January 1, 2010, through July 1, 2019. Accepted studies from the previous advisory were also rereviewed, covering the period of January 1, 1990, through July 31, 2010. Only studies containing original findings from peer-reviewed journals were acceptable. Editorials, letters, and other articles without data were excluded. A literature search strategy and PRISMA ††††††††† flow diagram are available as Supplemental Digital Content 2 ( https://links.lww.com/ALN/B980 ).
In total, 1,143 new citations were identified, with 810 articles assessed for eligibility. After review, 746 were excluded, with 24 new studies meeting the above stated criteria. These studies were combined with 40 pre-2010 articles used in the previous advisory and 8 provided by task force members, resulting in a total of 72 articles accepted as evidence for these guidelines. In this document, 63 peer-reviewed articles, 2 ASA standards, and 1 ASA practice advisory are referenced, with a complete bibliography of articles used to develop these guidelines, organized by section, available as Supplemental Digital Content 3 ( https://links.lww.com/ALN/B981 ).
Each pertinent outcome reported in a study was classified by evidence category and level and designated as beneficial, harmful, or equivocal. Findings were then summarized for each evidence linkage and reported in the text of the updated advisory, with evidence tables available as Supplemental Digital Content 4 ( https://links.lww.com/ALN/B982 ).
Evidence categories refer specifically to the strength and quality of the research design of the studies. Category A evidence represents results obtained from randomized controlled trials, and category B evidence represents observational results obtained from nonrandomized study designs or randomized controlled trials without pertinent comparison groups. When available, category A evidence is given precedence over category B evidence for any particular outcome. These evidence categories are further divided into evidence levels. Evidence levels refer specifically to the strength and quality of the summarized study findings ( i.e. , statistical findings, type of data, and the number of studies reporting/replicating the findings). In this document, only the highest level of evidence is included in the summary report for each intervention—outcome pair, including a directional designation of benefit, harm, or equivocality.
C ategory A. Randomized controlled trials report comparative findings between clinical interventions for specified outcomes. Statistically significant ( P < 0.01) outcomes are designated as either beneficial (B) or harmful (H) for the patient; statistically nonsignificant findings are designated as equivocal (E).
Level 1. The literature contains a sufficient number of randomized controlled trials to conduct meta-analysis, ‡‡‡‡‡‡‡‡‡ and meta-analytic findings from these aggregated studies are reported as evidence.
Level 2. The literature contains multiple randomized controlled trials, but the number of randomized controlled trials is not sufficient to conduct a viable meta-analysis for the purpose of these guidelines. Findings from these randomized controlled trials are reported separately as evidence.
Level 3. The literature contains a single randomized controlled trial, and findings from this study are reported as evidence.
C ategory B. Observational studies or randomized controlled trials without pertinent comparison groups may permit inference of beneficial or harmful relationships among clinical interventions and clinical outcomes. Inferred findings are given a directional designation of beneficial (B), harmful (H), or equivocal (E). For studies that report statistical findings, the threshold for significance is P < 0.01.
Level 1. The literature contains nonrandomized comparisons ( e.g. , quasiexperimental, cohort [prospective or retrospective], or case-control research designs) with comparative statistics between clinical interventions for a specified clinical outcome.
Level 2. The literature contains noncomparative observational studies with associative statistics ( e.g. , correlation, sensitivity, and specificity).
Level 3. The literature contains noncomparative observational studies with descriptive statistics ( e.g. , frequencies, percentages).
Level 4. The literature contains case reports.
Insufficient Literature. The lack of sufficient scientific evidence in the literature may occur when the evidence is either unavailable ( i.e. , no pertinent studies found) or inadequate. Inadequate literature cannot be used to assess relationships among clinical interventions and outcomes because a clear interpretation of findings is not obtained due to methodologic concerns ( e.g. , confounding of study design or implementation) or the study does not meet the criteria for content as defined in the “focus” of the guidelines.
Although interobserver agreement among task force members and two methodologists was not assessed for this update, the original guidelines reported agreement levels using a κ statistic for two-rater agreement pairs as follows: (1) type of study design, κ = 0.72 to 0.90; (2) type of analysis, κ = 0.80 to 0.90; (3) evidence linkage assignment, κ = 0.84 to 1.00; and (4) literature inclusion for database, κ = 0.70 to 1.00. Three-rater agreement values were as follows: (1) study design, Sav = 0.81, Var (Sav) = 0.010; (2) type of analysis, Sav = 0.86, Var (Sav) = 0.009; (3) linkage assignment, Sav = 0.82 Var (Sav) = 0.005; and (4) literature database inclusion Sav=0.78 Var (Sav) = 0.031. These values represent moderate to high levels of agreement.
Consensus-based Evidence Validation of the concepts addressed by this advisory and subsequent recommendations proposed was obtained by consensus from multiple sources, including: (1) survey opinions from consultants §§§§§§§§§ who were selected based on their knowledge or expertise in perioperative management of cardiac implantable electronic devices; (2) survey opinions from randomly selected samples of active members of the ASA; (3) testimony on the original advisory from attendees of two publicly held open forums at a national anesthesia meeting and at a major cardiology meeting; and (4) internet commentary. All opinion-based evidence relevant to each topic was considered in the development of these guidelines. However, only findings obtained from formal surveys are reported in the document. Opinion surveys were developed by the task force to address each clinical intervention identified in the document. Identical surveys were distributed to expert consultants and a random sample of members of the participating organizations.
Survey responses were recorded using a five-point scale and summarized based on median values. ‖‖‖‖‖‖‖‖‖
Strongly agree: Median score of 5 (at least 50% of the responses are 5)
Agree: Median score of 4 (at least 50% of the responses are 4 or 4 and 5)
Equivocal: Median score of 3 (at least 50% of the responses are 3, or no other response category or combination of similar categories contain at least 50% of the responses)
Disagree: Median score of 2 (at least 50% of responses are 2 or 1 and 2)
Strongly disagree: Median score of 1 (at least 50% of responses are 1)
The survey rate of return was 34% (N = 32/94) for consultants, and 5% (N = 245/5,000) for the ASA membership. The results of the surveys are reported in tables 2 and 3 and are summarized in the text of the guidelines. #########
An additional survey was sent to the consultants accompanied by a draft of the advisory asking them to indicate which, if any, of the evidence linkages would change their clinical practices if the advisory were instituted. The rate of return was 13% (N = 12 of 94). The percentage of responding consultants expecting no change associated with each linkage were as follows: preoperative evaluation (determining whether a patient has a cardiac implantable electronic device and that it is functioning properly), 83.3%; patient preparation (determining whether electromagnetic interference is likely to occur), 83.3%; consulting a specialist when needed to alter the pacing function of a cardiac implantable electronic device, 75.0%; having temporary pacing and defibrillation equipment immediately available before, during, and after procedures with electromagnetic interference potential, 91.7%; continuous monitoring of electrocardiography, Sp o 2 , and peripheral pulse, 91.7%; electrosurgery, 100%; radiofrequency ablation, 100%; lithotripsy, 91.7%; magnetic resonance imaging, 91.7%; radiation therapy, 100%; radiofrequency identification devices, 100%; electroconvulsive therapy, 100%; emergency defibrillation or cardioversion, 91.7%; postoperative management (continuing to monitor and display electrocardiogram, cardiac rate, and rhythm), 100%; postoperative management (for a cardiac implantable electronic device that was reprogrammed pre- or intraoperatively, restore the cardiac implantable electronic device to its permanent settings before the patient is discharged from a monitored environment), 83.3%; and postoperative cardiac implantable electronic device interrogation, 91.7%. In total, 67% of the respondents indicated that the advisory would have no effect on the amount of time spent on a typical case with the implementation of this advisory, 25% indicated that there would be an increase, and 8.3% indicated that there would be a decrease.
Acknowledgment
The authors are indebted to the late Dr. Marc A. Rozner for his service as the original chair of this Task Force and his invaluable contributions to the earlier versions of this advisory.
Research Support
Support was provided solely by the American Society of Anesthesiologists (Schaumburg, Illinois).
Competing Interests
The authors declare no competing interests.
NASPE/BPEG Generic Pacemaker Code: Revised (2002)

NASPE/BPEG Generic Defibrillator Code

Example of a Stepwise Approach to the Perioperative Management of the Patient with a Cardiac Implantable Electronic Device

Expert Consultant Survey Results

ASA Member Survey Results

Updated by the Committee on Standards and Practice Parameters: Jeffrey L. Apfelbaum, M.D. (Committee Chair), Chicago, Illinois; Peter M. Schulman, M.D. (Task Force Co-Chair), Portland, Oregon; Aman Mahajan, M.D., Ph.D. (Task Force Co-Chair), Pittsburgh, Pennsylvania; Richard T. Connis, Ph.D. (Chief Methodologist), Woodinville, Washington; and Madhulika Agarkar, M.P.H. (Methodologist), Schaumburg, Illinois.
Generic pacemaker and defibrillator codes are provided in tables 1 and 2 . Note that every transvenous implantable cardioverter–defibrillator includes both pacing and shock therapy capabilities for the management of bradyarrhythmias and tachyarrhythmias.
Inappropriate implantable cardioverter–defibrillator therapy refers to the delivery of antitachycardia therapy (pacing or shock) in the absence of a clinically indicated tachyarrhythmia. Inappropriate implantable cardioverter–defibrillator therapy can harm a patient by inducing ischemia, worsening the arrhythmia, or causing the patient to move during a delicate procedure.
More information about the management of patients with cardiac implantable electronic devices undergoing magnetic resonance imaging or radiation therapy may be found in the 2017 Heart Rhythm Society Expert Consensus Statement on Magnetic Resonance Imaging and Radiation Exposure in Patients with Cardiovascular Implantable Electronic Devices ( https://www.hrsonline.org/2017-hrs-expert-consensus-statement-cardiovascular-implantable-electronic-device-lead-management ; accessed August 9, 2019).
Not all implantable electronic devices are cardiac implantable electronic devices ( i.e. , deep brain stimulators, spinal cord stimulators, vagal nerve stimulators, gastric stimulators, phrenic nerve stimulators, etc. ). Although most cardiac implantable electronic device generators are in a pectoral position, some are in the abdomen or in an alternate position in the thorax ( i.e. , subcutaneous implantable cardioverter–defibrillator). Some cardiac implantable electronic devices are now implanted entirely within the heart ( i.e. , leadless pacemaker).
Many cardiac implantable electronic devices now have remote interrogation and monitoring capabilities. Thus, the most recent cardiac implantable electronic device interrogation report might be from an in-office interrogation or from a remote transmission (provided the remote transmission contains all needed information).
Most cardiac implantable electronic devices have an x-ray code inscribed on the generator that can be used to identify the cardiac implantable electronic device manufacturer.
A patient with an absent intrinsic heart rhythm is completely pacing-dependent. A patient with an inadequate intrinsic heart rhythm may be considered relatively or functionally pacing-dependent.
In many patients, determining proper cardiac implantable electronic device function can be accomplished by accessing the patient’s most recent cardiac implantable electronic device interrogation report. Note that the majority of consultants and ASA members agree that a cardiac implantable electronic device should be interrogated within 3 to 6 months before a procedure.
A cardiac implantable electronic device specialist might need to be consulted to help determine key information about the cardiac implantable electronic device, whether the patient is pacing-dependent, the cardiac implantable electronic device’s current settings, and that it is functioning properly.
If electromagnetic interference is unlikely, it may be unnecessary to alter the pacing function of a cardiac implantable electronic device to an asynchronous pacing mode. Altering the pacing function of a pacemaker to an asynchronous pacing mode may be accomplished by reprogramming or in many cases by applying a magnet. For most pacemakers, magnet application will initiate asynchronous pacing at a fixed pacing rate with a fixed atrioventricular delay. Some pacemakers have a programmable magnet response or no magnet response ( i.e. , some leadless pacemakers). Altering the pacing function of an implantable cardioverter–defibrillator to an asynchronous pacing mode must always be accomplished by reprogramming, because magnet application will never alter the pacing mode of an implantable cardioverter–defibrillator.
If electromagnetic interference is unlikely, it may be unnecessary to suspend the antitachycardia function of an implantable cardioverter–defibrillator. Suspending the antitachycardia function of an implantable cardioverter–defibrillator may be accomplished by reprogramming or in many cases by applying a magnet. A magnet correctly applied to an implantable cardioverter–defibrillator often results in suspension of antitachycardia therapy. For most implantable cardioverter–defibrillators, there is no reliable means to confirm the magnet response. Some implantable cardioverter–defibrillators may have no magnet response. In obese patients or those with a deep generator implant ( i.e. , subcutaneous implantable cardioverter–defibrillator), magnet application might fail to elicit the magnet response. The antitachycardia function of some older implantable cardioverter–defibrillators can be permanently disabled by magnet application.
Note that the majority of consultants disagree and ASA members are equivocal regarding the recommendation to alter the pacing function of a cardiac implantable electronic device to an asynchronous pacing mode in the pacing-dependent patient if monopolar electrosurgery (“bovie”) use is planned inferior to the umbilicus,
Suspending the active rate sensor of a pacemaker may be accomplished by reprogramming or in many cases by magnet application. Suspending the active rate sensor of an implantable cardioverter–defibrillator must always be accomplished by reprogramming.
The term “continuous” means “prolonged without any interruption at any time” (see Standards for Basic Anesthetic Monitoring, American Society of Anesthesiologists. Approved by the ASA House of Delegates October 21, 1986, and last amended October 28, 2015).
The peripheral pulse may be continuously monitored with either pulse oximetry plethysmography or an intraarterial pressure waveform.
Note that some cardiac implantable electronic devices are labeled by the Food and Drug Administration as magnetic resonance imaging-conditional. Any cardiac implantable electronic device system not labeled as such by the Food and Drug Administration is considered non–magnetic resonance imaging-conditional.
For some cases, the electrosurgical dispersive electrode will need to be placed at a site different from the thigh. For example, in head and neck cases, the dispersive electrode may be placed on the posterior superior aspect of the shoulder contralateral to the generator position.
An underbody electrosurgery dispersive electrode that is incorporated into a pad and placed directly on the operating table is sometimes used instead of a conventional dispersive electrode. In patients with a cardiac implantable electronic device, there is insufficient evidence to determine the impact of using an underbody dispersive electrode as compared with a conventional dispersive electrode on the risk of electromagnetic interference.
An inhibitory effect could occur even when the active electrode of the electrosurgery instrument is not touching the patient.
More information about the management of patients with cardiac implantable electronic devices undergoing magnetic resonance imaging or radiation therapy may be found in the 2017 Heart Rhythm Society Expert Consensus Statement on Magnetic Resonance Imaging and Radiation Exposure in Patients with Cardiovascular Implantable Electronic Devices ( https://www.hrsonline.org/2017-hrs-expert-consensus-statement-cardiovascular-implantable-electronic-device-lead-management ; accessed August 7, 2019).
Some cardiac implantable electronic devices are labeled by the Food and Drug Administration as magnetic resonance imaging-conditional. These systems have been approved for magnetic resonance imaging under specific conditions of use. Cardiac implantable electronic devices that do not meet these criteria are non–magnetic resonance imaging-conditional. In many centers, magnetic resonance imaging remains contraindicated in the presence of a magnetic resonance imaging nonconditional cardiac implantable electronic device; however, some centers have implemented specific protocols allowing patients with a nonconditional cardiac implantable electronic device to undergo magnetic resonance imaging.
Postoperative cardiac implantable electronic device interrogation may not be needed in low-risk situations ( e.g. , appropriate preoperative cardiac implantable electronic device interrogation, no electromagnetic interference-generating devices used during the procedure, no perioperative reprogramming occurred, and no problems identified during the procedure).
In some instances, new settings may be needed.
Although the antitachycardia function of some older implantable cardioverter–defibrillators can be permanently disabled by magnet application, these implantable cardioverter–defibrillators are unlikely to still be encountered.
If the cardiac implantable electronic device is not interrogated during the immediate postoperative period, an interrogation after the patient is discharged may be warranted. Note that the expert consultants strongly agree and ASA members agree that interrogation should occur within 30 days after a procedure.
Refer to table 3 for an example of a stepwise approach to the perioperative management of the patient with a cardiac implantable electronic device.
If electromagnetic interference is unlikely, it may be unnecessary to suspend the antitachycardia function of an implantable cardioverter–defibrillator. Suspending the antitachycardia function of an implantable cardioverter–defibrillator may be accomplished by reprogramming or in many cases by applying a magnet. A magnet correctly applied to an implantable cardioverter–defibrillator often results in suspension of antitachycardia therapy. For most implantable cardioverter–defibrillators, there is no reliable means to confirm the magnet response. Some implantable cardioverter–defibrillators may have no magnet response. In obese patients or those with a deep cardiac implantable electronic device implant ( i.e. , subcutaneous implantable cardioverter–defibrillator), magnet application might fail to elicit the magnet response. The antitachycardia function of some older implantable cardioverter–defibrillators can be permanently disabled by magnet application.
The term “continuous” means “prolonged without any interruption at any time” (see Standards for Basic Anesthetic Monitoring, American Society of Anesthesiologists; approved by the ASA House of Delegates October 21, 1986; last amended October 28, 2015).
Some cardiac implantable electronic devices are labeled by the Food and Drug Administration as magnetic resonance imaging-conditional. These systems have been approved for magnetic resonance imaging under specific conditions of use. Cardiac implantable electronic devices that do not meet these criteria are non–magnetic resonance imaging-conditional. In many centers, magnetic resonance imaging remains contraindicated in the presence of an non–magnetic resonance imaging-conditional cardiac implantable electronic device; however, some centers have implemented specific protocols allowing patients with a nonconditional cardiac implantable electronic device to undergo magnetic resonance imaging.
Preferred reporting items of systematic reviews and meta-analyses.
All meta-analyses are conducted by the ASA methodology group. Meta-analyses from other sources are reviewed but not included as evidence in this document. A minimum of five independent randomized controlled trials ( i.e. , sufficient for fitting a random-effects model) is required for meta-analysis.
Consultants were drawn from the following specialties where perioperative management of cardiac implantable electronic devices are a concern: anesthesiology (85% of respondents) and cardiac electrophysiology (15% of respondents).
When an equal number of categorically distinct responses are obtained, the median value is determined by calculating the arithmetic mean of the two middle values. Ties are calculated by a predetermined formula.
To view a bar chart with the above findings, refer to Supplemental Digital Content 5 ( https://links.lww.com/ALN/B983 ).
Citing articles via
Most viewed, email alerts, related articles, social media, affiliations.
- ASA Practice Parameters
- Online First
- Author Resource Center
- About the Journal
- Editorial Board
- Rights & Permissions
- Online ISSN 1528-1175
- Print ISSN 0003-3022
- Anesthesiology
- ASA Monitor

- Terms & Conditions Privacy Policy
- Manage Cookie Preferences
- © Copyright 2024 American Society of Anesthesiologists
This Feature Is Available To Subscribers Only
Sign In or Create an Account
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Pacemaker types and selection.
Hassan Mehmood Lak ; Amandeep Goyal .
Affiliations
Last Update: December 11, 2022 .
- Definition/Introduction
Pacemakers are electric activity generating devices used to treat patients with slow heart rate or symptomatic heart blocks and in patients with heart failure. [1] All cardiac pacemakers are generally composed of a pulse generator that generates the electrical current required for stimulation of heart musculature and one or two electrodes (also referred to as leads), which are responsible for transmitting the electrical activity generated by the pulse generator to the heart musculature. [2] [3]
- Issues of Concern
Common Indications for Pacemaker Placement
- Sinus node dysfunction (Class I indication) [4]
- Acquired AV block
- Post myocardial infarction [5]
Less Common Indications
- Congenital complete heart block
- Long QT syndrome
- Hypertrophic cardiomyopathy [6] [7]
- Heart failure
Conditions in Which Cardiac Pacing is Not Indicated
- Syncope of undetermined etiology
- Sinus bradycardia without significant symptoms
- Sinoatrial block or sinus arrest without significant symptoms
- Asymptomatic prolonged PR intervals with atrial fibrillation
- Asymptomatic bradycardia during sleep
- Asymptomatic 2nd-degree Mobitz I (Wenckebach ) AV block
- Right bundle branch block with left axis deviation without syncope or other symptoms compatible with intermittent AV block
- Reversible AV block such as those with electrolyte abnormalities, Lyme disease, sleep apnea
Pacemaker Related Complications
Pacemaker Syndrome: Pacemaker syndrome is a disease that represents the clinical consequences of suboptimal atrioventricular (AV) synchrony or AV dyssynchrony, regardless of the pacing mode after pacemaker implantation.
Symptoms of Pacemaker Syndrome are:
- Cannon A-waves
- Palpitations
- Shortness of breath
Other complications associated with Pacemakers include:
- Pneumothorax
- Cardiac perforation
- Significant pocket hematoma
- Lead dislodgement
- Venous thrombosis and obstruction
- Mechanical lead complications
- Clinical Significance
Pacemaker Types and Systems
Pulse Generators: These are the "battery" component of the pacemaker, which normally produces the electrical activity required to transmit to the heart musculature. Pulse generators are currently placed most commonly in the infraclavicular region of the anterior chest wall.
Trans venous Systems : Most of the cardiac pacing systems use the transvenous electrodes to transmit electrical impulses from the pulse generator to the heart musculature.
Epicardial systems : These work by direct stimulation through the pulse generator by attaching directly to the heart's surface. They are less common use nowadays, and transvenous pacing has completely replaced them.
Leadless systems : There have been some newer innovations to develop leadless systems due to some limitations with transvenous and epicardial pacing systems. [8] [9] [10]
Types of Pacemakers
There are three basic kinds of pacemakers:
- Single chamber . One lead attaches to the upper or lower heart chamber.
- Dual-chamber . Uses two leads, one for the upper and one for the lower chamber
- Biventricular pacemakers (used in cardiac resynchronization therapy).
Modes of Cardiac Pacing
The modes of pacemakers are based on generic code known as NBG ( combined from NASPE/BPEG) and typically consist of 5 letters. [11]
- Letter 1. The area being paced, A stands for atria, V stands for Ventricle, D stands for Dual, O stands for none
- Letter 2. The area which is sensed, A stands for atria, V stands for Ventricle, D stands for Dual, O stands for none
- Letter 3. The response of the pacemaker to sensing: O stands for none, I stands for inhibiting, T stands for triggering, D stands for dual
- Letter 4. Rate adaptiveness. O stands for none, R stands for rate adaptiveness.
The modes are explainable by dividing them into categories of a single chamber or dual chamber:
Single Chamber Modes
V- Pacing in the ventricle
O- Sensing is OFF
O- Response to sensing is OFF
In this mode, the pacemaker paces at a programmed rate regardless of the intrinsic electrical activity of the heart.
V- Sensing in the ventricle
In this mode, the pacemaker can sense the electrical activity and withhold pacing when not required.
- AOO
A- Pacing in the atrium
A- Pacing in the atrium
A- Sensing in the atrium
I- Inhibit
In this mode, the pacemaker can adapt to the intrinsic atrial rate and should be able to pace when needed and inhibit when not required.
Dual Chamber Modes
Dual Chamber Modes can further subdivide into Tracking Modes and Non-Tracking modes.
Tracking Modes
D- Pacing in the atrium and ventricle
D- Sensing in the atrium and ventricle
D- Inhibit and or trigger
Intrinsic P-wave and QRS can inhibit pacing, and intrinsic P-wave or atrial pace can trigger an AV delay.
This mode is fully capable of adapting to intrinsic heart rhythm and mimicking normal conduction as much as possible.
DDD has four distinct pacing patterns
- AsVs (Atrial sensed ventricle sensed): Used when the patient has good sinus node function and good AV node function
- AsVp (Atrial sensed Ventricular paced): Used when the patient has a good sinus node function but poor AV node conduction
- ApVs (Atrial paced Ventricular sensed): Used when the patient has poor sinus node function but has intact AV node conduction
- ApVp (Atrial paced Ventricular paced): Used when the patient has a poor function in both the sinus node and AV node.
Intrinsic QRS can inhibit ventricular pacing, and Intrinsic P-wave can trigger an AV delay.
In this mode, one cannot pace the atrium, but an intrinsic atrial activity can trigger an AV delay resulting in P-wave tracking and possibly maintaining AV synchrony.
The primary issue with VDD programming mode is that if sinus node function drops below the pacemaker programmed lower rate; then it will cause AV dissociation due to the inability to pace in the atrium.
VDD mode should only be used in patients with good SA node function. It might be used in a situation where the patient has a high pacing threshold in the atrium. This way, the pacemaker will be able to sense in the atrium, maintain AV synchrony, and not waste battery life by pacing in the high threshold atrium.
Non-Tracking Modes
D - Pacing in the atrium and ventricle
D - Sensing in the atrium and ventricle
I - Response to that sensing will be to either pace or inhibit
This mode's primary use is in patients with atrial tachyarrhythmias and mode switch algorithms. DDI mode will result in AV dissociation if the atrial rate goes high than the set rate. P wave tracking is excellent for AV synchrony; however, if the patient goes into atrial fibrillation with rapid heart rate, one does not want to track the atrium and pace the ventricle at a high rate.
O- Response to that sensing is OFF
This mode results in AV sequential pacing at the lower rate limit regardless of the heart's own intrinsic activity. DOO mode is asynchronous pacing and is usually used only in certain situations, such as when a magnet is placed over a pacemaker or sometimes when a patient is having surgery.
R- Rate Response
Rate Response or Rate Adaptive Pacing is used in patients with chronotropic incompetence. Chronotropic incompetence is defined as the inability of the heart to appropriately increase its rate with increased activity or metabolic demand that leads to exercise intolerance. Usually, there is a problem with SA node function.
The pacemaker utilizes its sensing ability with the aid of sensors that can sense motion or minute ventilation changes according to the activity and pace the heart at a required rate.
Choosing a Pacing Mode
While selecting a pacemaker mode, the clinician will want to ensure that the patient has a viable atrial activity. If the atrium is healthy, the clinician will want to maintain AV synchrony as much as possible.
The first important question is if the sinus node function is intact?
If sinus node function is not intact and the patient has atrial arrhythmias:
Chronic Atrial Arrhythmias, e.g., Atrial Fibrillation or Atrial Flutter:
- If the patient is chronotropically incompetent, the mode of choice will be VVIR.
- If the patient is chronotropically competent, the mode of choice will be VVI.
Paroxysmal Atrial Arrhythmias:
- If the patient is chronotropically incompetent, the mode of choice will be DDDR.
- If the patient is chronotropically competent, the mode of choice will be DDD.
If the sinus node is intact and the patient has normal sinus rhythm or sinus bradycardia:
Intact AV Node conduction
- If the patient is chronotropically incompetent, the mode of choice will be AAIR.
- If the patient is chronotropically competent, the mode of choice will be AAI.
AV Node conduction not Intact:
- If the patient is chrontropically incompetent, the mode of choice will be DDDR.
- Nursing, Allied Health, and Interprofessional Team Interventions
Pacemakers are implanted in situations when the intrinsic electrical activity of the heart is either dyssynchronous and the patient is having symptomatic episodes resulting from the asynchronous electrical impulse stimulated from the SA node. Different pacemaker types and modes, as mentioned above, are available, and a deep and close monitoring and understanding of these modes and close-loop monitoring by the inter-professional team is of utmost importance in the management of patients with cardiac pacemakers.
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Disclosure: Hassan Mehmood Lak declares no relevant financial relationships with ineligible companies.
Disclosure: Amandeep Goyal declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Lak HM, Goyal A. Pacemaker Types and Selection. [Updated 2022 Dec 11]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Biventricular pacing (cardiac resynchronization therapy): an evidence-based analysis. [Ont Health Technol Assess Ser....] Biventricular pacing (cardiac resynchronization therapy): an evidence-based analysis. Medical Advisory Secretariat. Ont Health Technol Assess Ser. 2005; 5(13):1-60. Epub 2005 Sep 1.
- Present day pacemakers for pulse generator exchange: is 3.5 V a sufficient nominal setting for the pulse amplitude? Thera Pacemaker Study Group. [Pacing Clin Electrophysiol. 1996] Present day pacemakers for pulse generator exchange: is 3.5 V a sufficient nominal setting for the pulse amplitude? Thera Pacemaker Study Group. Schuchert A, Van Langen H, Michels K, Meinertz T. Pacing Clin Electrophysiol. 1996 Nov; 19(11 Pt 2):1824-7.
- Can transcutaneous electrical nerve stimulation be safely used in patients with permanent cardiac pacemakers? [Mayo Clin Proc. 1988] Can transcutaneous electrical nerve stimulation be safely used in patients with permanent cardiac pacemakers? Rasmussen MJ, Hayes DL, Vlietstra RE, Thorsteinsson G. Mayo Clin Proc. 1988 May; 63(5):443-5.
- Review Smart Pacemaker: A Review. [Cureus. 2022] Review Smart Pacemaker: A Review. Agarwal S, Shinde RK. Cureus. 2022 Oct; 14(10):e30027. Epub 2022 Oct 7.
- Review Artificial cardiac pacemakers. [Int J Clin Pharmacol Ther Toxi...] Review Artificial cardiac pacemakers. Das G, Carlblom D. Int J Clin Pharmacol Ther Toxicol. 1990 May; 28(5):177-89.
Recent Activity
- Pacemaker Types and Selection - StatPearls Pacemaker Types and Selection - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
- To save this word, you'll need to log in. Log In
wandering pacemaker
Medical Definition of wandering pacemaker
Dictionary entries near wandering pacemaker.
wandering cell
Wangensteen apparatus
Cite this Entry
“Wandering pacemaker.” Merriam-Webster.com Medical Dictionary , Merriam-Webster, https://www.merriam-webster.com/medical/wandering%20pacemaker. Accessed 3 May. 2024.
Subscribe to America's largest dictionary and get thousands more definitions and advanced search—ad free!

Can you solve 4 words at once?
Word of the day.
See Definitions and Examples »
Get Word of the Day daily email!
Popular in Grammar & Usage
What’s the difference between ‘hillbilly’ and ‘redneck’, more commonly misspelled words, commonly misspelled words, how to use em dashes (—), en dashes (–) , and hyphens (-), absent letters that are heard anyway, popular in wordplay, 12 star wars words, the words of the week - may 3, 9 superb owl words, 10 words for lesser-known games and sports, your favorite band is in the dictionary, games & quizzes.


COMMENTS
Wandering atrial pacemaker (WAP) is an atrial rhythm where the pacemaking activity of the heart originates from different locations within the atria. This is different from normal pacemaking activity, where the sinoatrial node (SA node) is responsible for each heartbeat and keeps a steady rate and rhythm. Causes of wandering atrial pacemaker are unclear, but there may be factors leading to its ...
A wandering atrial pacemaker is a rare form of a condition called arrhythmia. That's a problem with your heartbeat. It can happen anytime, even when you're sleeping. It's usually nothing to ...
With wandering atrial pacemaker, the ECG shows variable P-wave morphology and PR intervals. The atrial impulses conduct in a 1:1 fashion and usually control the rhythm for several beats before ...
Wandering Atrial Pacemaker (WAP) is a cardiac rhythm disorder that causes irregular and variable heartbeats. Learn the Heart - Healio provides a comprehensive ECG review of this condition ...
This article is a guide for interpreting abnormal Wandering Atrial Pacemaker EKGs, including qualifying criteria and a sample EKG rhythnm strip. Wandering atrial pacemaker is an arrhythmia originating in shifting pacemaker sites from the SA node to the atria and back to the SA node. On an ECG, the p-waves reflect the pacemaker shifts by shape variations. The PRI interval may vary from one beat ...
This rhythm and multifocal atrial tachycardia are similar except for heart rate. The other possible explanation is that there is significant respiratory sinus arrhythmia, with uncovering of latent foci of pacemaker activity. Usually, it is associated with underlying lung disease. In the elderly, it may be a manifestation of sick sinus syndrome.
Three or more ectopic foci within the atrial myocardium serve as the pacemaker; Rate is less than 100bpm (in contrast to MAT) Is irregularly irregular therefore sometimes confused with atrial fibrillation and sinus arrhythmia; Causes. Intrinsic cardiac or pulmonary disease; Metabolic derangements; Drug toxicity (including Digoxin) Clinical Features
Wandering Pacemaker. Wandering pacemaker. Every p-wave is different and thus has a different origin. When several pacemakers are competing, p-waves with different origins and thus configurations occur. The rhythm is slightly different from beat to beat. note If the heart rate increases to above 100bpm, it is called Multifocal Atrial Tachycardia.
Wandering atrial pacemaker (WAP) is a benign atrial arrhythmia observed in elderly patients suffering from obstructive pulmonary diseases that result from an ischemic heart. This report discusses WAP as observed in a patient who suffered an electrical injury. Keywords: wandering atrial pacemaker, voltage, electrical injury, arrhythmia, ampere.
Figure 1. Imaging of the Migrated Temporary Epicardial Pacemaker Wire. (A) Transesophageal echocardiogram revealing a linear density (W) in the left ventricle (LV) attached to the mitral valve (MV) traveling through the bioprosthetic aortic valve (AV) and into the ascending aorta (A). (B) Computed tomography angiogram showing the 15-cm density ...
Diagnose Wandering Atrial Pacemaker with confidence! This video explores the shifting pacemaker sites and the resulting "multifocal P waves" observed on an E...
Cardiac pacemakers are effective treatments for a variety of bradyarrhythmias. By providing an appropriate heart rate and heart rate response, cardiac pacing can reestablish effective circulation and normalize hemodynamics that are compromised by a slow heart rate. This topic will present a broad review of the role of cardiac pacing in a ...
Wandering Pacemaker. To the Editor: An electrocardiographic pattern of irregular, multiform (multifocal), supraventricular beats with changing P wave morphology and varying P-R intervals has been referred to as wandering pacemaker. This term has been discouraged by some because it implies a mechanism which is not really known.
The rate tends to increase on inspiration and decrease with expiration because of changes in vagal tone. Often, there is an accompanying change in P wave configuration (wandering pacemaker) with the P waves becoming taller and spiked during inspiration and flatter in expiration.
Wandering Atrial Pacemaker is aptly named due to the electrical impulses causing the atrial activity are moving or wandering. These changes in the locus of stimulation affect the morphology of the P waves. Analysis. In Wandering Atrial Pacemaker ECG, you must observe at least three different shaped P waves. No other changes in the tracing may ...
ABSTRACT. Temporary cardiac pacing is commonly used in patients with life-threatening bradycardia and serves as a bridge to implantation of a permanent pacemaker (PPM).For years, passive fixation leads have been used for this purpose, offering the advantage of that they can be placed at bedside. The downside, however, is that patients must remain on telemetry and bed rest until lead removal ...
Wandering Atrial Pacemaker Rhythm Strip Features. Rate: Normal (60-100 bpm) Rhythm: May be irregular. P Wave: Changing shape and size from beat to beat (at least three different forms) PR Interval: Variable. QRS: Normal (0.06-0.10 sec) The electrical impulses causing the atrial activity are moving or wandering.
This practice advisory updates the "Practice Advisory for the Perioperative Management of Patients with Cardiac Implantable Electronic Devices: Pacemakers and Implantable Cardioverter-Defibrillators: An Updated Report by the American Society of Anesthesiologists Task Force on Perioperative Management of Patients with Cardiac Implantable Electronic Devices," adopted by the American ...
Ellis W. Lader MD, FACC, FAHA, FACP. Search for more papers by this author. Book Author(s):
heart block had an epicardial single-chamber pacemaker implanted at 2 days of age. At 21 months of age, while sitting or standing, the patient's right anterior thigh muscles contracted at her pulse rate. Surgical exploration revealed a free-floating pacemaker in her peritoneum. A new dual-chamber pacemaker was implanted into the
Pacemakers are electric activity generating devices used to treat patients with slow heart rate or symptomatic heart blocks and in patients with heart failure.[1] All cardiac pacemakers are generally composed of a pulse generator that generates the electrical current required for stimulation of heart musculature and one or two electrodes (also referred to as leads), which are responsible for ...
The meaning of WANDERING PACEMAKER is a back-and-forth shift in the location of cardiac pacemaking especially from the sinoatrial node to or near the atrioventricular node.
This week Dr. O'Sullivan discusses a wandering pacemaker on EKG. Obi Veterinary Education - Veterinary CE made easy. For more content visit www.obivet.comOu...